Question: Problem 2. A liquid containing 35 mole % benzene and 65 mole % 2-propanol is continuously fed to and distilled in a flash drum at

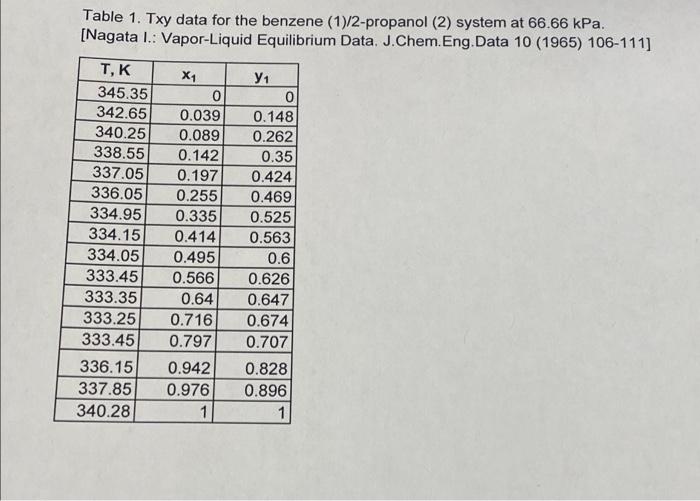
Problem 2. A liquid containing 35 mole % benzene and 65 mole % 2-propanol is continuously fed to and distilled in a flash drum at 101.3 kPa. The feed rate is 100 kg moles/hr. Equilibrium data is given in Table 1. Print the operating lines from parts (a) - (c) on the same diagram and label each line. Printing the diagram and labeling each operating line by hand is allowed. a. If 24 % of the feed remains as liquid, what are the vapor and liquid mole fractions for benzene, and the total vapor (V) and liquid (L) flow rates? Show the operating line on the xy diagram and plot the diagram in mole fractions of benzene. Show the equation for the operating line with numerical values for the slope and the intercept b. If the desired liquid product is 30 mole % benzene, what vapor fraction (V/F) should be used? Determine the total product flow rates (V and L) and mole fraction of benzene in the vapor phase. Show the operating line on the xy diagram. c. If the drum temperature is 334.95 K, find the mole fractions of benzene in the liquid and vapor products (using Txy data) and calculate V/F. Show the operating line on the xy diagram. Table 1. Txy data for the benzene (1)/2-propanol (2) system at 66.66 kPa. [Nagata 1.: Vapor-Liquid Equilibrium Data. J. Chem. Eng. Data 10 (1965) 106-111] TEK 345.35 342.65 340.25 338.55 337.05 336.05 334.95 334.15 334.05 333.45 333.35 333.25 333.45 336.15 337.85 340.28 X1 0 0.039 0.089 0.142 0.197 0.255 0.335 0.414 0.495 0.566 0.64 0.716 0.797 Y1 0 0.148 0.262 0.35 0.424 0.469 0.525 0.563 0.6 0.626 0.647 0.674 0.707 0.828 0.896 1 0.942 0.976 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


