Question: Problem 2. Dimethylcarbonate (DMC, C3H6O3 ) is a reactant in the process to synthesize polycarbonates, which are used in a wide variety of products including
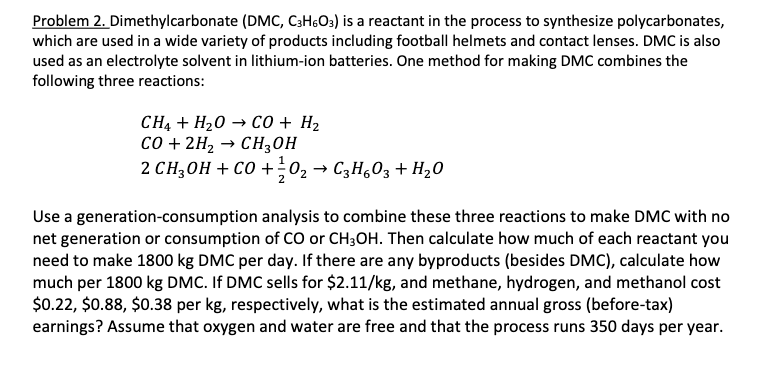
Problem 2. Dimethylcarbonate (DMC, C3H6O3 ) is a reactant in the process to synthesize polycarbonates, which are used in a wide variety of products including football helmets and contact lenses. DMC is also used as an electrolyte solvent in lithium-ion batteries. One method for making DMC combines the following three reactions: CH4+H2OCO+H2CO+2H2CHH3OH2CH3OH+CO+21O2C3H6O3+H2O Use a generation-consumption analysis to combine these three reactions to make DMC with no net generation or consumption of CO or CH3OH. Then calculate how much of each reactant you need to make 1800kg DMC per day. If there are any byproducts (besides DMC), calculate how much per 1800kg DMC. If DMC sells for $2.11/kg, and methane, hydrogen, and methanol cost $0.22,$0.88,$0.38 per kg, respectively, what is the estimated annual gross (before-tax) earnings? Assume that oxygen and water are free and that the process runs 350 days per year
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


