Question: Problem 2: Ethylene Oxide production Stream table for production of ethylene oxide. All flows in gmol/s Ethylene oxide, C2H4O, (EO) is produced in 4 world-scale
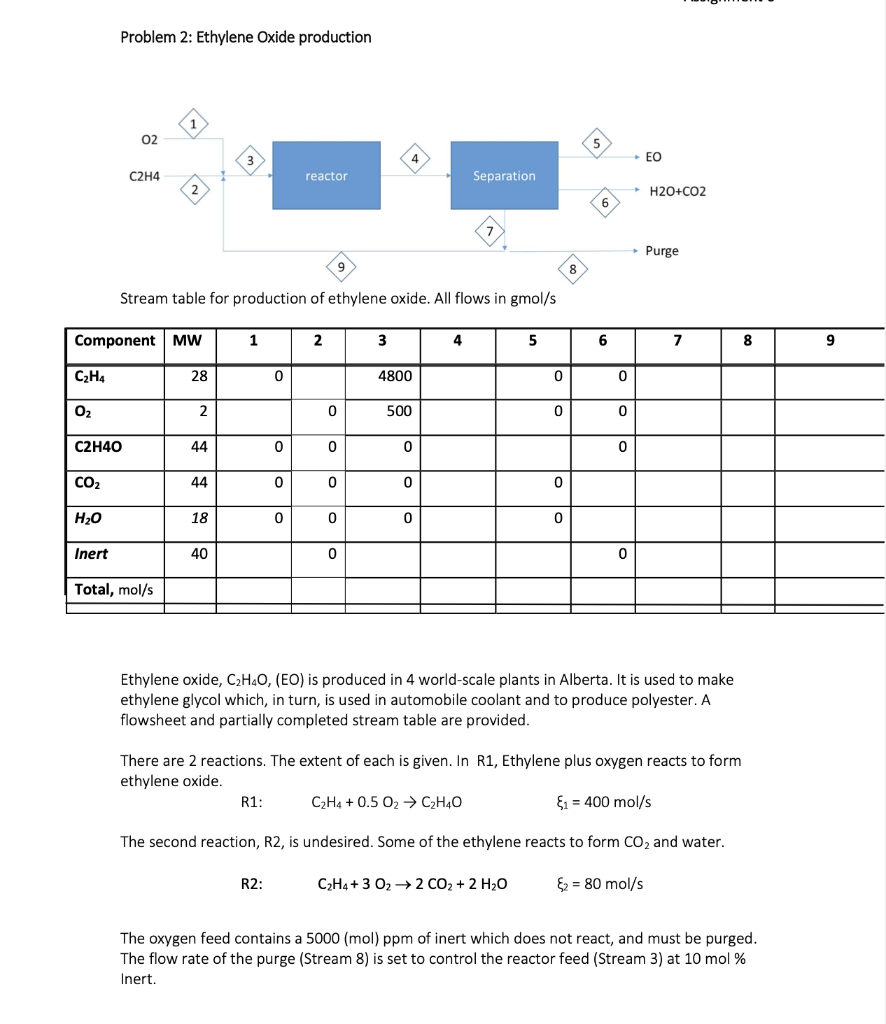

Problem 2: Ethylene Oxide production Stream table for production of ethylene oxide. All flows in gmol/s Ethylene oxide, C2H4O, (EO) is produced in 4 world-scale plants in Alberta. It is used to make ethylene glycol which, in turn, is used in automobile coolant and to produce polyester. A flowsheet and partially completed stream table are provided. There are 2 reactions. The extent of each is given. In R1, Ethylene plus oxygen reacts to form ethylene oxide. R1:C2H4+0.5O2C2H4O1=400mol/s The second reaction, R2, is undesired. Some of the ethylene reacts to form CO2 and water. R2:C2H4+3O22CO2+2H2O2=80mol/s The oxygen feed contains a 5000 (mol) ppm of inert which does not react, and must be purged. The flow rate of the purge (Stream 8) is set to control the reactor feed (Stream 3) at 10mol% Inert. Questions for problem 2. a) What is the limiting reactant? Explain, including equations. b) Determine the molar flow rate inert in the reactor feed (stream 3) and molar flow rates of all reactor components in the reactor effluent (stream 4) and enter them into the stream table. c) What is the fractional conversion of oxygen in the reactor? d) What is the Selectivity of the reactor? e) Use the information provided above to complete the stream table. Complete the stream table on the page, and attach it to your assignment. f) Demonstrate an overall carbon balance for the process. If it does not balance, explain. g) How much (expensive) ethylene is lost in the purge, expressed as a fraction of the feed ethylene? How much (expensive) ethylene is lost due to reaction 2, expressed as a fraction of the ethylene feed? If you were trying to improve the overall process yield, where would you focus your efforts? h) What is the reactor yield of ethylene to ethylene oxide? The overall process yield of ethylene to EO and oxygen to EO? You have calculated 3 different 'yields'. They should be quite different. Explain the differences
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


