Question: Problem 2: The gas-phase decomposition A B+2C is carried out in a constant-volume batch reactor. Runs 1 through 5 were carried out at 100
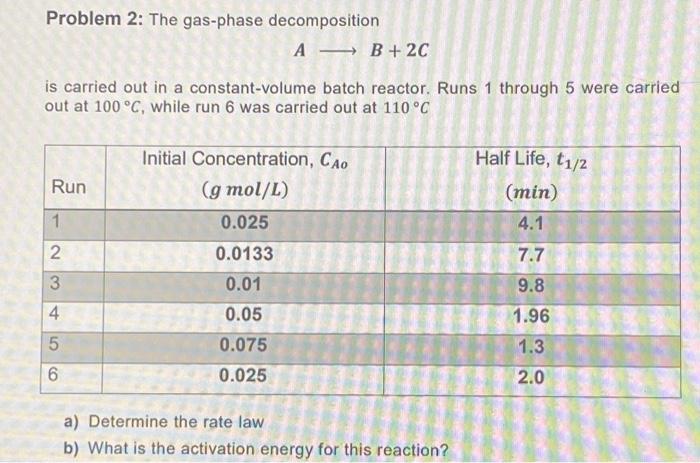
Problem 2: The gas-phase decomposition A B+2C is carried out in a constant-volume batch reactor. Runs 1 through 5 were carried out at 100 C, while run 6 was carried out at 110 C Initial Concentration, Co Half Life, t1/2 Run (g mol/L) (min) 1 0.025 4.1 2 0.0133 7.7 3 0.01 9.8 4 0.05 1.96 5 0.075 1.3 6 0.025 2.0 a) Determine the rate law b) What is the activation energy for this reaction?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


