How would the answer change if the initial concentration of glyceryl stearate were 3 mol/dm 3 ?
Question:
How would the answer change if the initial concentration of glyceryl stearate were 3 mol/dm3? Rework Example 4-2 correctly using the information given in the problem statement.
Example 4-2
Having set up the stoichiometric table in Example 4-1, one can now readily use it to calculate the concentrations at a given conversion. If the initial mixture consists of sodium hydroxide at a concentration of 10 mol/dm3 (i.e., 10 mol/L or 10 kmol/m3) and glyceryl stearate at a concentration of 2 mol/dm3, what are the concentrations of glycerol stearate, B, and of glycerine, D, when the conversion of sodium hydroxide is
(a)
(b) 90%?
A 2-dimensional rhombus shown with four divisions and different shades represents the diamond levels of Nitrogen Tetroxide. The level of health hazard is 3, fire and instability hazards are 0, and OX is a specific hazard.
Table From Example 4-1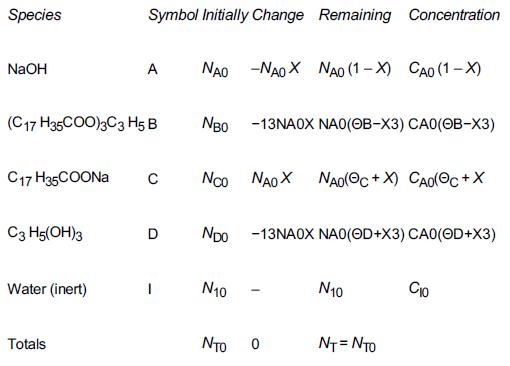
Step by Step Answer:






