Question: Problem 2. The reaction between CO and OH appears to be a simple bimolecular process: OH + co k1, H + CO2 [R 1] where
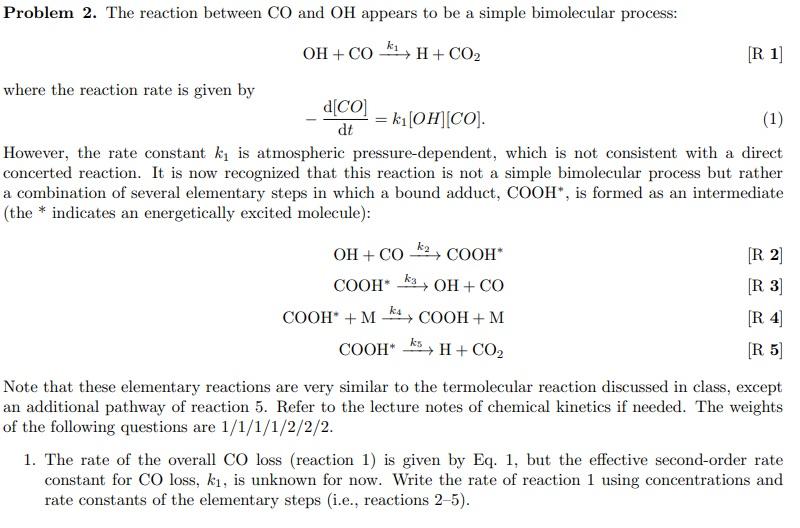
Problem 2. The reaction between CO and OH appears to be a simple bimolecular process: OH + co k1, H + CO2 [R 1] where the reaction rate is given by d[CO] = k1OH (CO). (1) dt However, the rate constant ki is atmospheric pressure-dependent, which is not consistent with a direct concerted reaction. It is now recognized that this reaction is not a simple bimolecular process but rather a combination of several elementary steps in which a bound adduct, COOH", is formed as an intermediate (the * indicates an energetically excited molecule): OH + CO2, COOH [R2 COOH KOH + CO [R 3 COOH +M K+ COOH+M [ R4 COOH H + CO2 [R 5 Note that these elementary reactions are very similar to the termolecular reaction discussed in class, except an additional pathway of reaction 5. Refer to the lecture notes of chemical kinetics if needed. The weights of the following questions are 1/1/1/1/2/2/2. 1. The rate of the overall CO loss (reaction 1) is given by Eq. 1, but the effective second-order rate constant for CO loss, k, is unknown for now. Write the rate of reaction 1 using concentrations and rate constants of the elementary steps i.e., reactions 25). k4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


