Question: Problem 2. The reactions listed below are taking place in a porous slab with length L. Both reactions are first order and irreversible. The
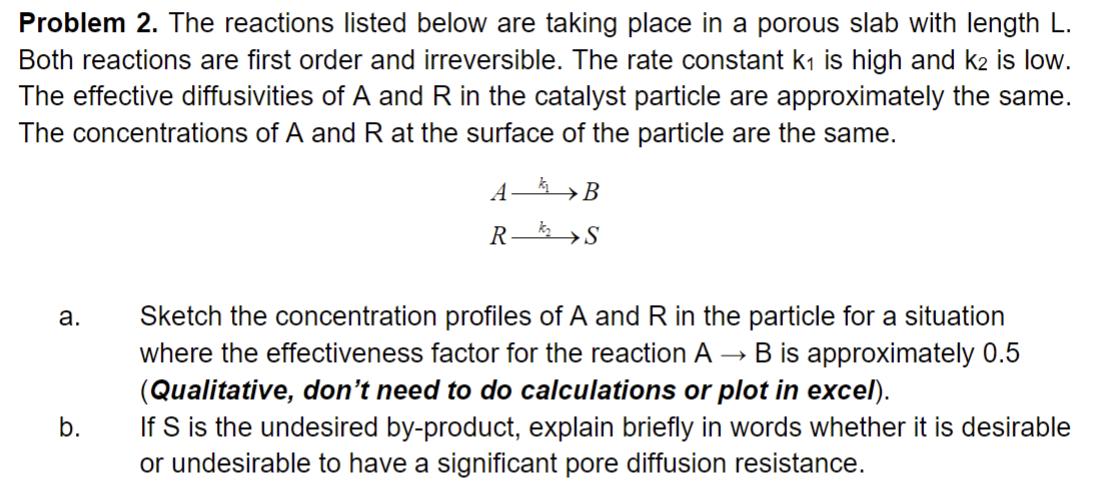
Problem 2. The reactions listed below are taking place in a porous slab with length L. Both reactions are first order and irreversible. The rate constant k is high and k2 is low. The effective diffusivities of A and R in the catalyst particle are approximately the same. The concentrations of A and R at the surface of the particle are the same. AB R S a. b. Sketch the concentration profiles of A and R in the particle for a situation where the effectiveness factor for the reaction A B is approximately 0.5 (Qualitative, don't need to do calculations or plot in excel). If S is the undesired by-product, explain briefly in words whether it is desirable or undesirable to have a significant pore diffusion resistance.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


