Question: Problem 2. You perform a second degradation experiment in which you incubate PLGA (50:50) pellets of known mass in buffer at pH=7.4 for a given
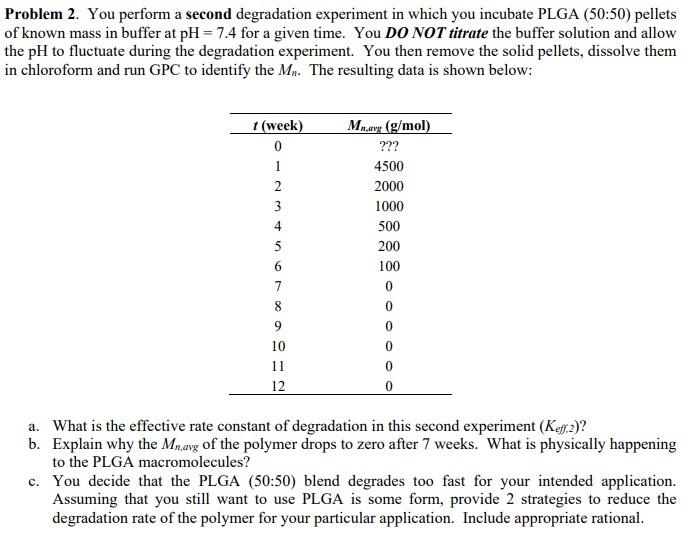
Problem 2. You perform a second degradation experiment in which you incubate PLGA (50:50) pellets of known mass in buffer at pH=7.4 for a given time. You DO NOT titrate the buffer solution and allow the pH to fluctuate during the degradation experiment. You then remove the solid pellets, dissolve them in chloroform and run GPC to identify the Mn. The resulting data is shown below: a. What is the effective rate constant of degradation in this second experiment (Keff,2) ? b. Explain why the Mn,avg of the polymer drops to zero after 7 weeks. What is physically happening to the PLGA macromolecules? c. You decide that the PLGA (50:50) blend degrades too fast for your intended application. Assuming that you still want to use PLGA is some form, provide 2 strategies to reduce the degradation rate of the polymer for your particular application. Include appropriate rational
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


