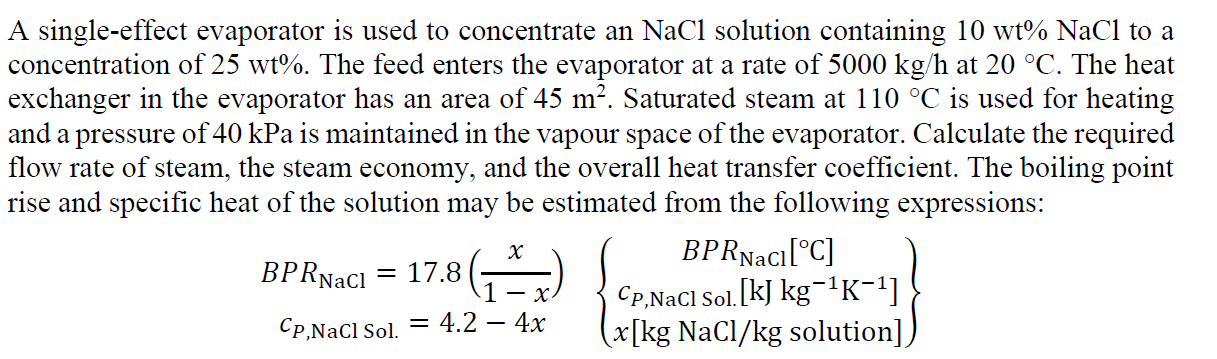
Question: Problem 2.8 A single-effect evaporator is used to concentrate an NaCl solution containing 10 wt% NaCl to a concentration of 25 wt%. The feed enters
 Problem 2.8
Problem 2.8
A single-effect evaporator is used to concentrate an NaCl solution containing 10 wt% NaCl to a concentration of 25 wt%. The feed enters the evaporator at a rate of 5000 kg/h at 20 C. The heat exchanger in the evaporator has an area of 45 m. Saturated steam at 110 C is used for heating and a pressure of 40 kPa is maintained in the vapour space of the evaporator. Calculate the required flow rate of steam, the steam economy, and the overall heat transfer coefficient. The boiling point rise and specific heat of the solution may be estimated from the following expressions: BPRNac[C] = CP,NaCl Sol. [kJ kg-1K-1] Cp,NaCl Sol. 4.2 4x (x[kg NaCl/kg solution] BPRwaci = 17.8(** - X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


