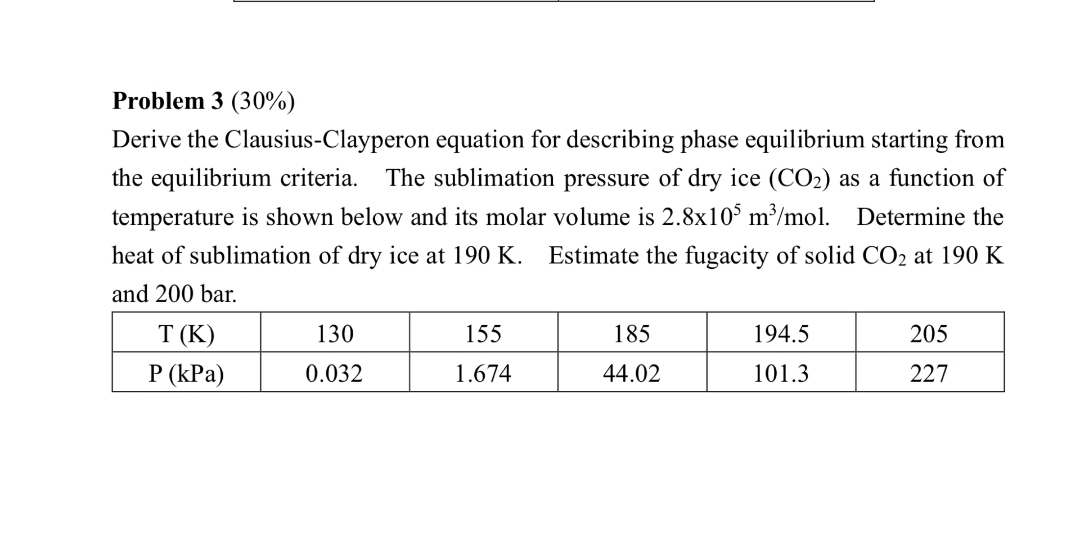
Question: Problem 3 ( 3 0 % ) Derive the Clausius - Clayperon equation for describing phase equilibrium starting from the equilibrium criteria. The sublimation pressure
Problem
Derive the ClausiusClayperon equation for describing phase equilibrium starting from
the equilibrium criteria. The sublimation pressure of dry ice as a function of
temperature is shown below and its molar volume is Determine the
heat of sublimation of dry ice at Estimate the fugacity of solid at
and bar.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


