Question: Problem 3. (40 points total, 3a and 3b) Lactose is broken down into monosaccharides by the following reaction: lactose(l) + H2O(l) glucose(l) + galactose(1) AH


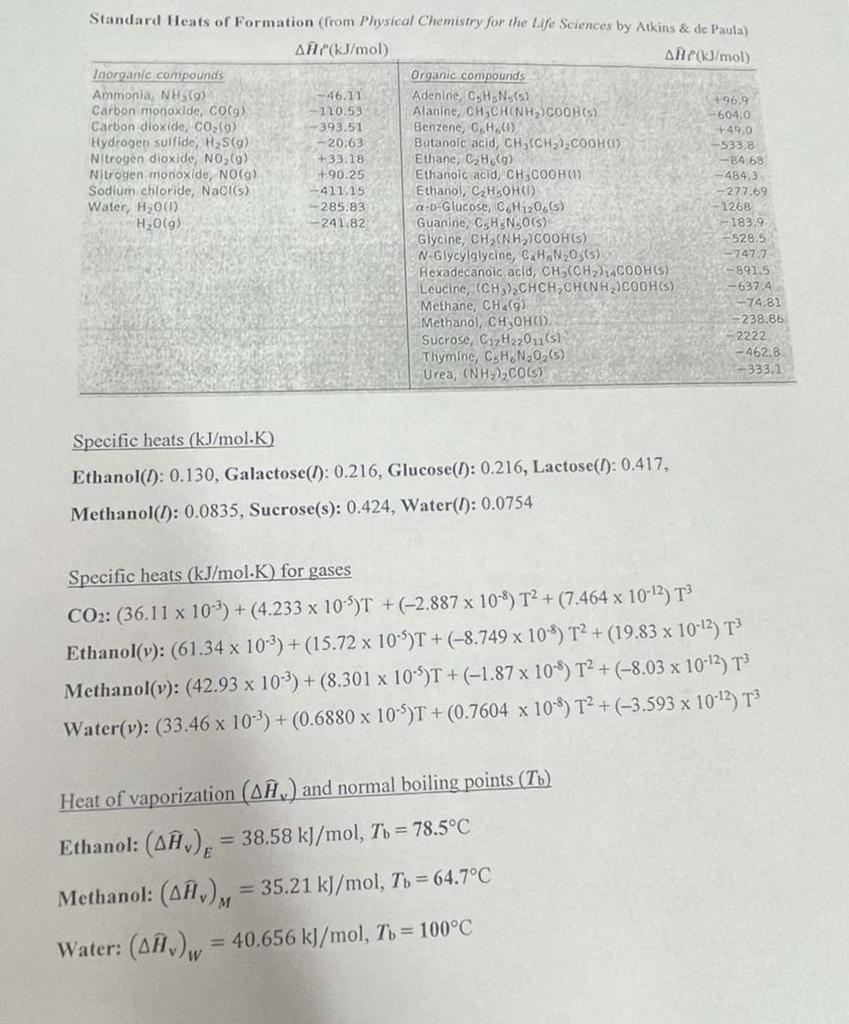
Problem 3. (40 points total, 3a and 3b) Lactose is broken down into monosaccharides by the following reaction: lactose(l) + H2O(l) glucose(l) + galactose(1) AH = 41.8 kJ The above reaction occurs in a bioreactor operated at steady-state with its contents mixed using an impeller which consumes power at the rate of 120 kJ/h. The feed to the bioreactor is lactose dissolved in water at 15C and 1 atm. The products and unreacted lactose leave together in an aqueous solution at 25C and 1 atm. (See figure below.) Impeller 4 moles lactose/h, 500 mols water/h 15C, I atm 3.2 moles glucose/h, 3.2 moles galactose/h 0.8 moles lactose/h, 496.8 mols water/h 25C, 1 atm Bioreactor 3a. Make an Enthalpy Table, with your reference states identified on top of the table. 3a Ar what rate is heat added to or removed from the bioreactor (identify which), in kan? -393.51 Standard Heats of Formation (from Physical Chemistry for the Life Sciences by Atkins & de Paula) AH(kJ/mol ANPJ/mol Inorganic compounds Organic compounds Ammonia, NH (9) -46.11 Adenine, CHN (5) +96.9 Carbon monoxide, COGG) 110.53 Alanine, CH SCHONHYCOOHS) -6040 Carbon dioxide, CO (9) Benzene, C.H.(1) +49.0 Hydrogen sulfide, HS(9) -- 20.63 Butanoic acid, CH, (CH2)2COOH(O) -533.8 Nitrogen dioxide, NOG +33.18 Ethane, CH (9) -84.68 Nitrogen monoxide, NO(g) +90.25 Ethanoic acid, CH3COOH) Sodium chloride, NaCl(s) ana Ethanol, CxH5OHD Water, H2001) -285.83 a-b Glucose, CH 120.65) -1268 H2O(9) -241.82 Guanine - 183.9 Glycine N30(5) CH, (NH2)COOH) -528.5 N-Glycylglycine, C.H.N203(5) -747.7 Hexadecanoic acid, CH,(CH2) CODHS) -891.5 Leucine, (CH)CHCH,CHINH)COOH(S) -637.4 Methane, CH.) -74.82 Methanol, CH OHOD -238.86 Sucrose, C2H22011(5) Thymine, C.H.N.O.(5) - 462,8 Urea, (NH3),COL) -333.1 -411.15 -484.3 -277.69 -2222 Specific heats (kJ/mol.K) Ethanol(1): 0.130, Galactose(l): 0.216, Glucose(t): 0.216, Lactose(): 0.417, Methanol(1): 0.0835, Sucrose(s): 0.424, Water(I): 0.0754 Specific heats (kJ/mol.K) for gases CO2: (36.11 x 10-2) +(4.233 x 10-5)T + (-2.887 x 10-9) T2 + (7.464 x 10-12) T3 Ethanol(y): (61.34 x 10-3) + (15.72 x 10-4)T + (-8.749 x 108) T2 + (19.83 x 10-42) T Methanol(v): (42.93 x 10-3) +(8.301 x 10-4)T + (-1.87 x 10-8) T2 + (-8.03 x 10-12) T3 Water(v): (33.46 x 10-2) + (0.6880 x 10-5)T + (0.7604 x 10-4) T2 + (-3.593 x 10-13) T3 Heat of vaporization (Aw) and normal boiling points (T6) Ethanol: (A), = 38.58 kJ/mol, To = 78.5C Methanol: (AA), = 35.21 kJ/mol, Tu = 64.7C Water: (AH), = 40.656 kJ/mol, Tu = 100C W
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


