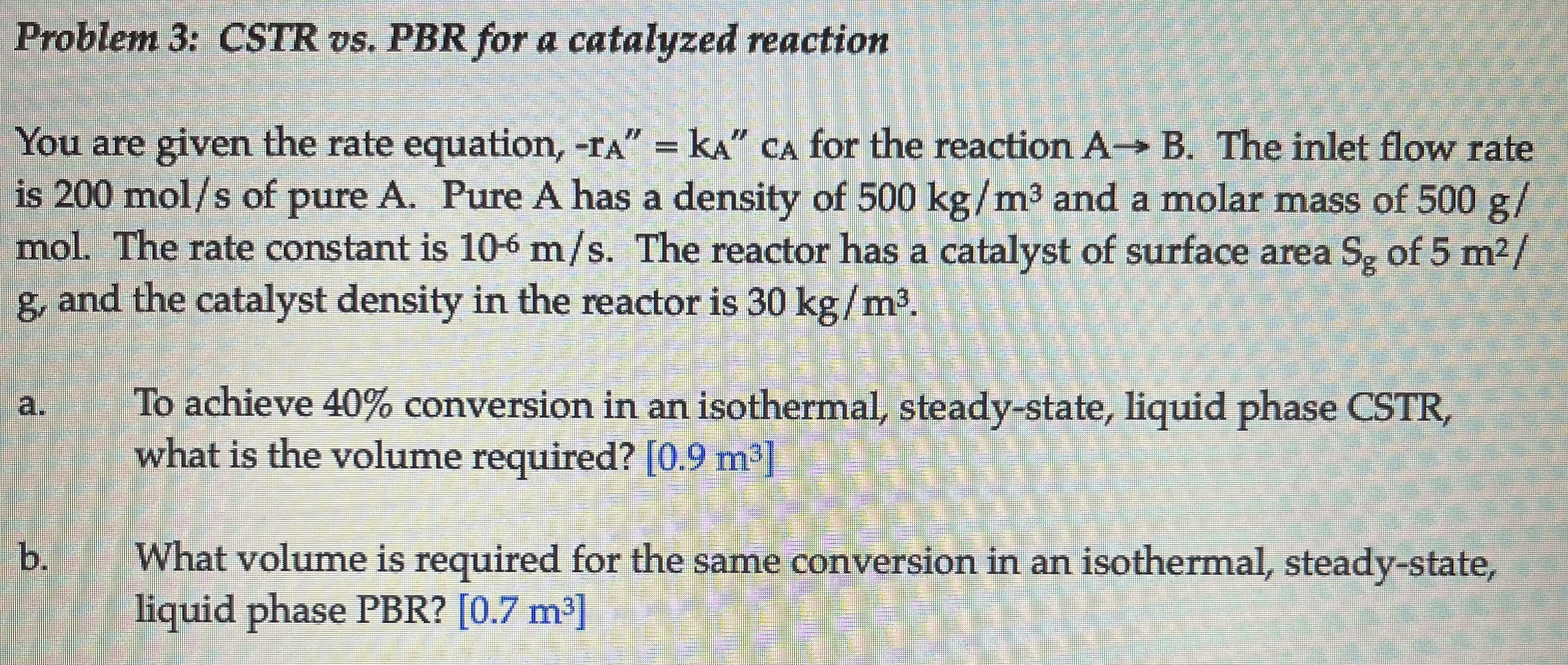
Question: Problem 3 : CSTR vs . PBR for a catalyzed reaction You are given the rate equation, - rA = kA cA for the reaction
Problem : CSTR vs PBR for a catalyzed reaction
You are given the rate equation, rA kA cA for the reaction A B The inlet flow rate
is mols of pure A Pure A has a density of kgm and a molar mass of g
mol. The rate constant is ms The reactor has a catalyst of surface area Sg of m
g and the catalyst density in the reactor is kgm
a To achieve conversion in an isothermal, steadystate, liquid phase CSTR
what is the volume required? m
b What volume is required for the same conversion in an isothermal, steadystate,
liquid phase PBR mProblem : CSTR vs PBR for a catalyzed reaction
You are given the rate equation, for the reaction The inlet flow rate
is of pure A Pure A has a density of and a molar mass of
mol. The rate constant is The reactor has a catalyst of surface area of
and the catalyst density in the reactor is
a To achieve conversion in an isothermal, steadystate, liquid phase CSTR
what is the volume required?
b What volume is required for the same conversion in an isothermal, steadystate,
liquid phase PBR m
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


