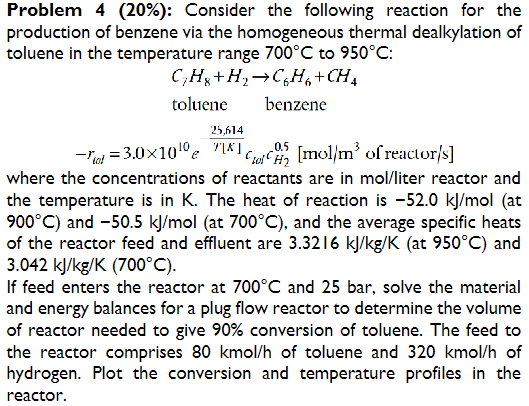
Question: Problem 4 ( 2 0 % ) : Consider the following reaction for the production of benzene via the homogeneous thermal dealkylation of toluene in
Problem : Consider the following reaction for the
production of benzene via the homogeneous thermal dealkylation of
toluene in the temperature range to :
toluene benzene
reactor
where the concentrations of reactants are in molliter reactor and
the temperature is in The heat of reaction is at
and at and the average specific heats
of the reactor feed and effluent are at and
If feed enters the reactor at and bar, solve the material
and energy balances for a plug flow reactor to determine the volume
of reactor needed to give conversion of toluene. The feed to
the reactor comprises kmo of toluene and kmo of
hydrogen. Plot the conversion and temperature profiles in the
reactor.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


