Question: Problem 5 (25 points). Consider the elementary reactions [ begin{array}{c} A stackrel{k_{1}}{ ightarrow} B B+C stackrel{k_{2}}{ ightarrow} D end{array} ] In a constant volume
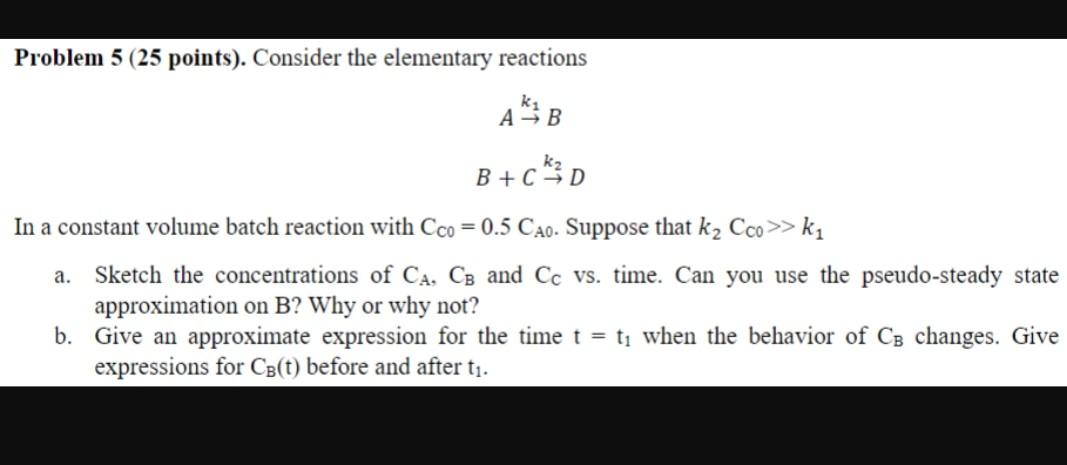
Problem 5 (25 points). Consider the elementary reactions \[ \begin{array}{c} A \stackrel{k_{1}}{ ightarrow} B \\ B+C \stackrel{k_{2}}{ ightarrow} D \end{array} \] In a constant volume batch reaction withCC0=0.5CA0. Suppose thatk2CC0k1a. Sketch the concentrations ofCA,CBandCCvs. time. Can you use the pseudo-steady state approximation onB? Why or why not? b. Give an approximate expression for the timet=t1when the behavior ofCBchanges. Give expressions forCB(t)before and aftert1.
Problem 5 (25 points). Consider the elementary reactions Ak1BB+Ck2D In a constant volume batch reaction with CC0=0.5CA0. Suppose that k2CC0>k1 a. Sketch the concentrations of CA,CB and CC vs. time. Can you use the pseudo-steady state approximation on B ? Why or why not? b. Give an approximate expression for the time t=t1 when the behavior of CB changes. Give expressions for CB(t) before and after t1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


