Question: Problem 5. Given a batch reactor with a series reaction in which species A reacts reversibly to form the desired species B. In this reaction,
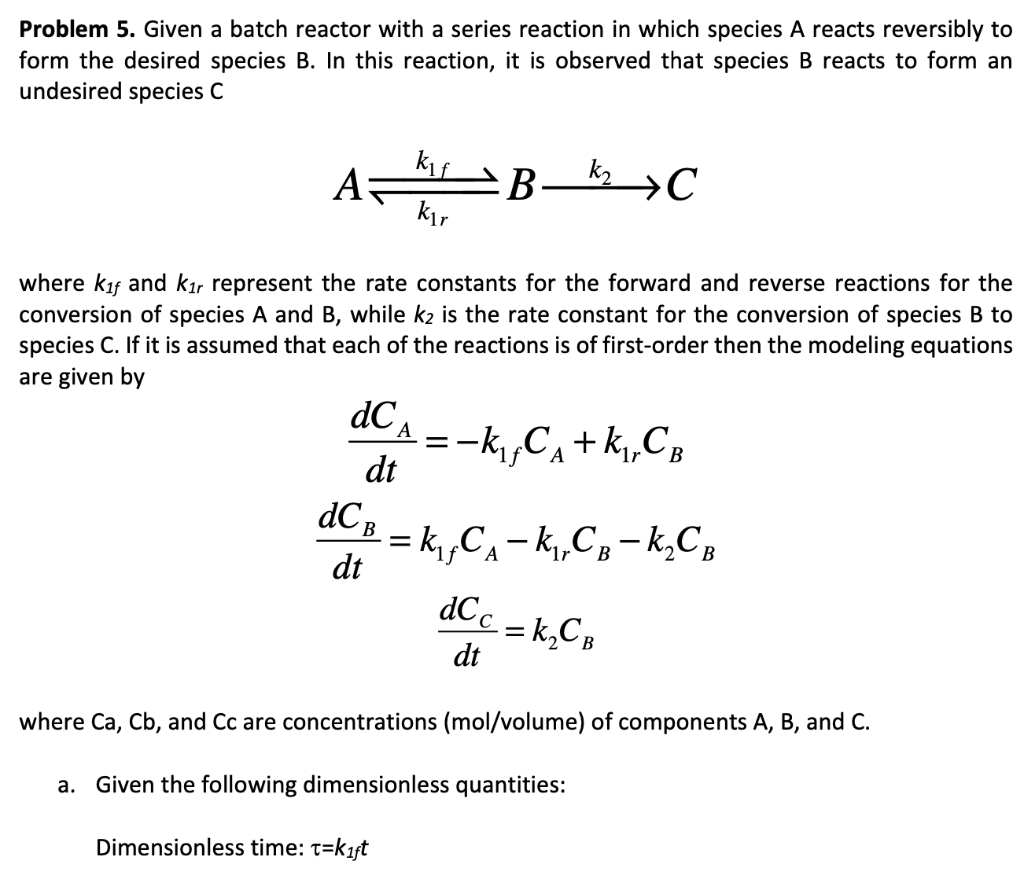

Problem 5. Given a batch reactor with a series reaction in which species A reacts reversibly to form the desired species B. In this reaction, it is observed that species B reacts to form an undesired species C kif A B-ky C kr where kf and kr represent the rate constants for the forward and reverse reactions for the conversion of species A and B, while k2 is the rate constant for the conversion of species B to species C. If it is assumed that each of the reactions is of first-order then the modeling equations are given by dCA =- =-k,CA+ k,,CB dt dCB = k,,Cx - k,Co-k,CA = A 1r B dt dCc = k CB dt where Ca, Cb, and Cc are concentrations (mol/volume) of components A, B, and C. a. Given the following dimensionless quantities: Dimensionless time: t=kift Conversion of species A: X1=(Cao-CA)/CAO Dimensionless concentration of species B: X2=CB/Cao Rate of rate coefficients:a=kz/kf Ratio of forward and reverse rate coefficients:=kr/kif Show that the equation for the dimensionless concentration of species B is: dX2 dx, +(a+B+1) 2 +aX2 = 0 dt? dt =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


