Question: Problem 5: Graph It! ( 3 points total) A mixture of n-hexane and ethyl alcohol is contained inside a rigid vessel at a pressure of
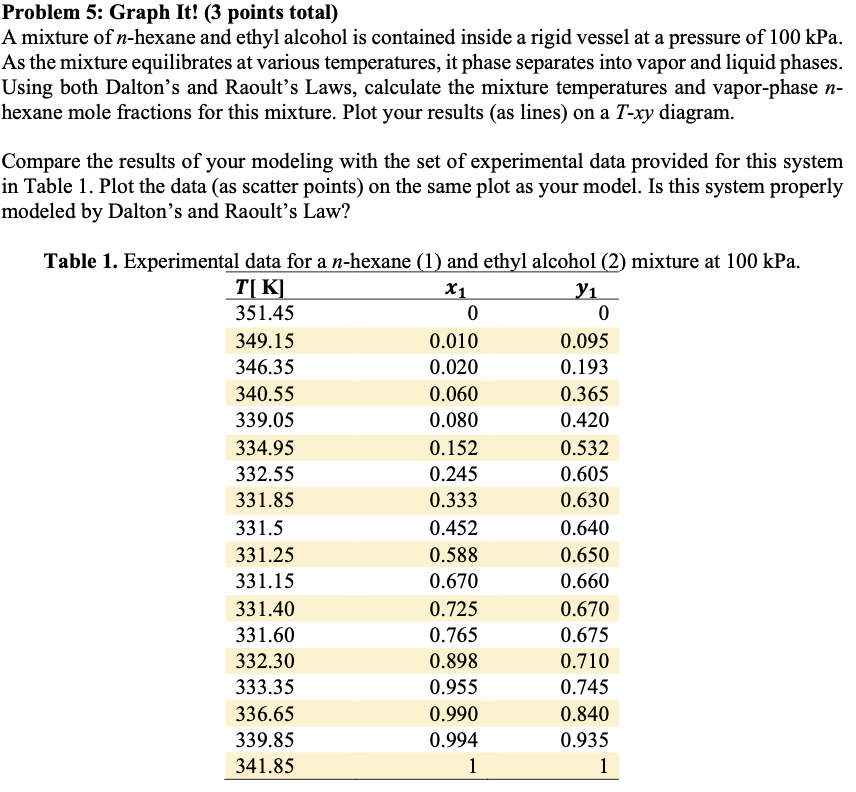
Problem 5: Graph It! ( 3 points total) A mixture of n-hexane and ethyl alcohol is contained inside a rigid vessel at a pressure of 100kPa. As the mixture equilibrates at various temperatures, it phase separates into vapor and liquid phases. Using both Dalton's and Raoult's Laws, calculate the mixture temperatures and vapor-phase n hexane mole fractions for this mixture. Plot your results (as lines) on a T-xy diagram. Compare the results of your modeling with the set of experimental data provided for this system in Table 1. Plot the data (as scatter points) on the same plot as your model. Is this system properly modeled by Dalton's and Raoult's Law
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


