Question: Problem 6 . Assume ethane combustion in air: C 2 H 6 + 7 2 O 2 = 2 C O 2 + 3 H
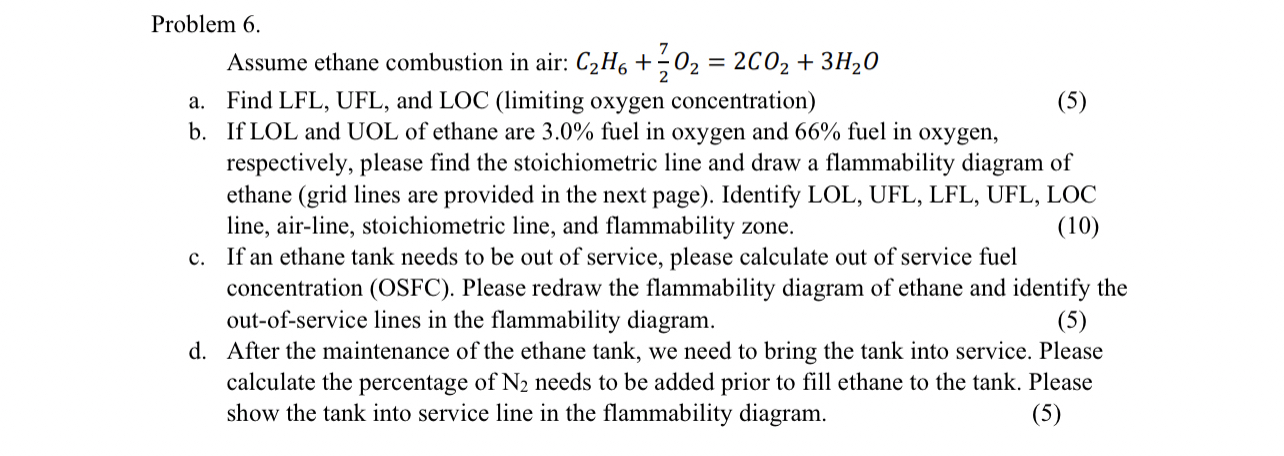
Problem
Assume ethane combustion in air:
a Find LFL UFL, and LOC limiting oxygen concentration
b If LOL and UOL of ethane are fuel in oxygen and fuel in oxygen, respectively, please find the stoichiometric line and draw a flammability diagram of ethane grid lines are provided in the next page Identify LOL, UFL, LFL UFL, LOC line, airline, stoichiometric line, and flammability zone.
c If an ethane tank needs to be out of service, please calculate out of service fuel concentration OSFC Please redraw the flammability diagram of ethane and identify the outofservice lines in the flammability diagram.
d After the maintenance of the ethane tank, we need to bring the tank into service. Please calculate the percentage of needs to be added prior to fill ethane to the tank. Please show the tank into service line in the flammability diagram.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


