Question: Problem 6.1 Table 6.10 gives oscillatory shear data taken at 170,180 , and 190C for a nearly monodisperse polystyrene having Mw=5.7104. You are asked to
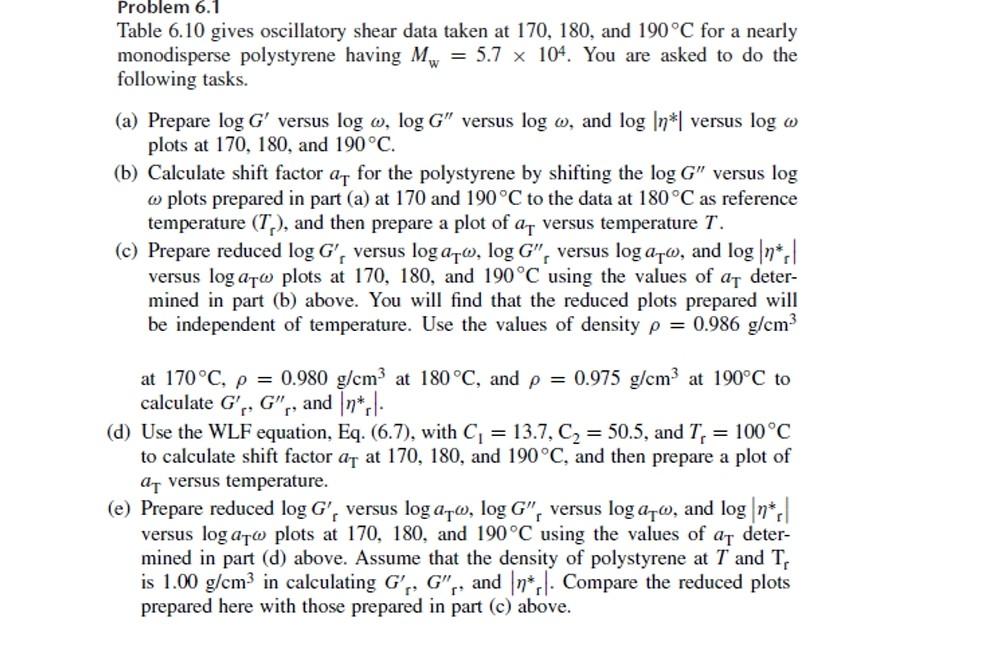
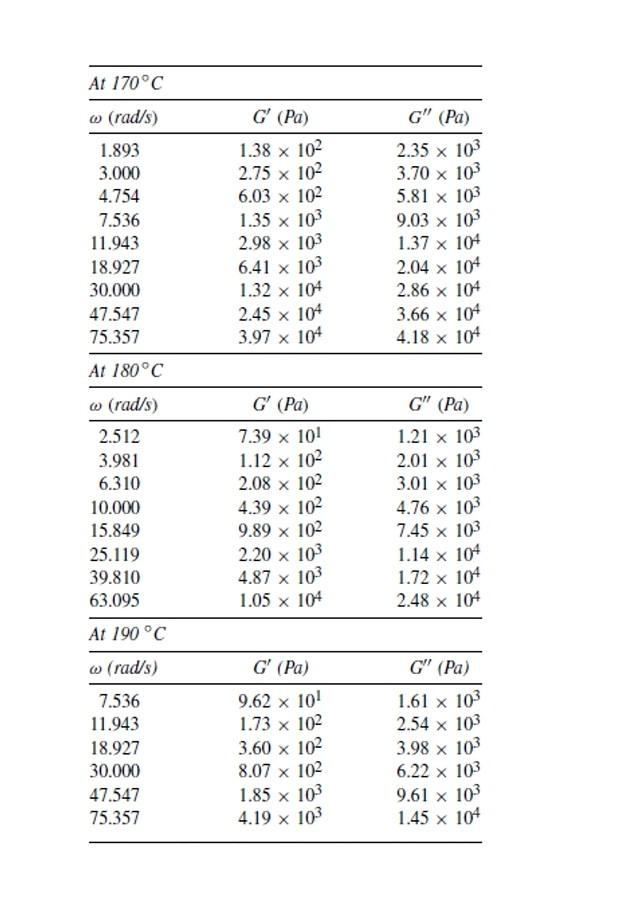
Problem 6.1 Table 6.10 gives oscillatory shear data taken at 170,180 , and 190C for a nearly monodisperse polystyrene having Mw=5.7104. You are asked to do the following tasks. (a) Prepare logG versus log,logG versus log, and log versus log plots at 170,180 , and 190C. (b) Calculate shift factor aT for the polystyrene by shifting the logG versus log plots prepared in part (a) at 170 and 190C to the data at 180C as reference temperature (Tr), and then prepare a plot of aT versus temperature T. (c) Prepare reduced logGr versus logaT,logGr versus logaT, and logr versus logaT plots at 170,180 , and 190C using the values of aT determined in part (b) above. You will find that the reduced plots prepared will be independent of temperature. Use the values of density =0.986g/cm3 at 170C,=0.980g/cm3 at 180C, and =0.975g/cm3 at 190C to calculate Gr,Gr, and rr. (d) Use the WLF equation, Eq. (6.7), with C1=13.7,C2=50.5, and Tr=100C to calculate shift factor aT at 170,180 , and 190C, and then prepare a plot of aT versus temperature. (e) Prepare reduced logGr versus logaT,logGr versus logaT, and logr versus logaT plots at 170,180 , and 190C using the values of aT determined in part (d) above. Assume that the density of polystyrene at T and Tr is 1.00g/cm3 in calculating Gr,G, and r. Compare the reduced plots prepared here with those prepared in part (c) above. \begin{tabular}{ccc} \hline At 170C & & \\ \hline(rad/s) & G(Pa) & G(Pa) \\ \hline 1.893 & 1.38102 & 2.35103 \\ 3.000 & 2.75102 & 3.70103 \\ 4.754 & 6.03102 & 5.81103 \\ 7.536 & 1.35103 & 9.03103 \\ 11.943 & 2.98103 & 1.37104 \\ 18.927 & 6.41103 & 2.04104 \\ 30.000 & 1.32104 & 2.86104 \\ 47.547 & 2.45104 & 3.66104 \\ 75.357 & 3.97104 & 4.18104 \\ \hline At 180C & & \\ \hline(rad/s) & G(Pa) & G(Pa) \\ \hline 2.512 & 7.39101 & 1.21103 \\ 3.981 & 1.12102 & 2.01103 \\ 6.310 & 2.08102 & 3.01103 \\ 10.000 & 4.39102 & 4.76103 \\ 15.849 & 9.89102 & 7.45103 \\ 25.119 & 2.20103 & 1.14104 \\ 39.810 & 4.87103 & 1.72104 \\ 63.095 & 1.05104 & 2.48104 \\ \hline At 190C & & \\ \hline(rad/s) & G(Pa) & G(Pa) \\ \hline 7.536 & 9.62101 & 1.61103 \\ 11.943 & 1.73102 & 2.54103 \\ 18.927 & 3.60102 & 3.98103 \\ 30.000 & 8.07102 & 6.22103 \\ 47.547 & 1.85103 & 9.61103 \\ 75.357 & 4.19103 & 1.45104 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


