Question: = Problem 8.1. Two liquids (A and B) are in chemical equilibrium according to: A + 2B The reduced excess Gibbs energy of the liquid
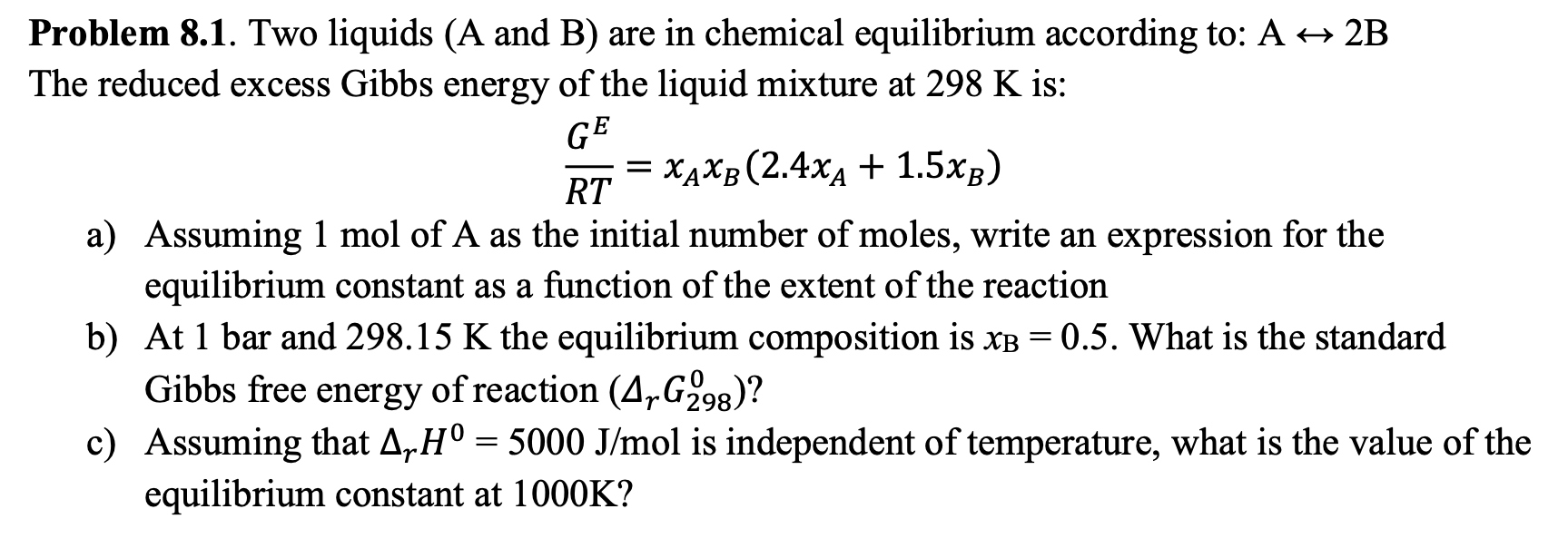
= Problem 8.1. Two liquids (A and B) are in chemical equilibrium according to: A + 2B The reduced excess Gibbs energy of the liquid mixture at 298 K is: GE = XAxb (2.4x4 + 1.5xb) RT a) Assuming 1 mol of A as the initial number of moles, write an expression for the equilibrium constant as a function of the extent of the reaction b) At 1 bar and 298.15 K the equilibrium composition is xB = 0.5. What is the standard Gibbs free energy of reaction (4,G293)? c) Assuming that ArH = 5000 J/mol is independent of temperature, what is the value of the equilibrium constant at 1000K? =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


