Question: Problem 8-6 (Level 2) Methyl cyclohexane (MCH) is being dehydrogenated to toluene (T) in a catalytic, fluidized-bed reactor. The feed to the reactor is a
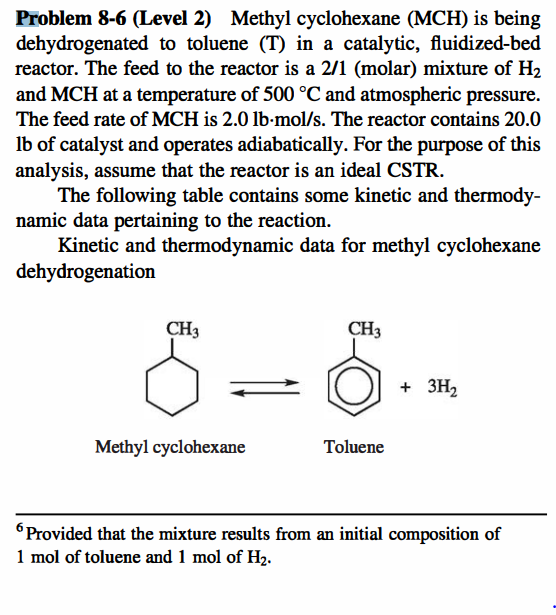
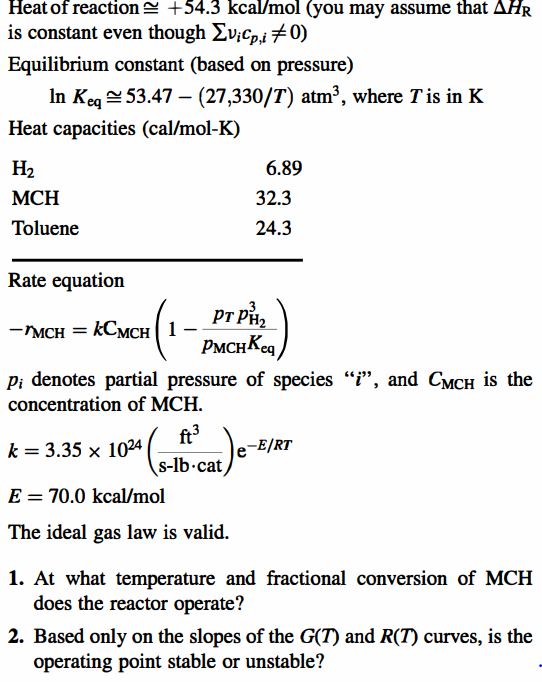
Problem 8-6 (Level 2) Methyl cyclohexane (MCH) is being dehydrogenated to toluene (T) in a catalytic, fluidized-bed reactor. The feed to the reactor is a 2/1 (molar) mixture of H2 and MCH at a temperature of 500C and atmospheric pressure. The feed rate of MCH is 2.0lbmol/s. The reactor contains 20.0 lb of catalyst and operates adiabatically. For the purpose of this analysis, assume that the reactor is an ideal CSTR. The following table contains some kinetic and thermodynamic data pertaining to the reaction. Kinetic and thermodynamic data for methyl cyclohexane dehydrogenation Methyl cyclohexane Toluene 6 Provided that the mixture results from an initial composition of 1mol of toluene and 1mol of H2. Heat of reaction +54.3kcal/mol (you may assume that HR is constant even though vicp,i=0 ) Equilibrium constant (based on pressure) lnKeq53.47(27,330/T)atm3, where T is in K Heat capacities (cal/mol-K) Rate equation rMCH=kCMCH(1pMCHKeqpTpH23) pi denotes partial pressure of species " i ", and CMCH is the concentration of MCH. k=3.351024(slbcatft3)eE/RT E=70.0kcal/mol The ideal gas law is valid. 1. At what temperature and fractional conversion of MCH does the reactor operate? 2. Based only on the slopes of the G(T) and R(T) curves, is the operating point stable or unstable? Problem 8-6 (Level 2) Methyl cyclohexane (MCH) is being dehydrogenated to toluene (T) in a catalytic, fluidized-bed reactor. The feed to the reactor is a 2/1 (molar) mixture of H2 and MCH at a temperature of 500C and atmospheric pressure. The feed rate of MCH is 2.0lbmol/s. The reactor contains 20.0 lb of catalyst and operates adiabatically. For the purpose of this analysis, assume that the reactor is an ideal CSTR. The following table contains some kinetic and thermodynamic data pertaining to the reaction. Kinetic and thermodynamic data for methyl cyclohexane dehydrogenation Methyl cyclohexane Toluene 6 Provided that the mixture results from an initial composition of 1mol of toluene and 1mol of H2. Heat of reaction +54.3kcal/mol (you may assume that HR is constant even though vicp,i=0 ) Equilibrium constant (based on pressure) lnKeq53.47(27,330/T)atm3, where T is in K Heat capacities (cal/mol-K) Rate equation rMCH=kCMCH(1pMCHKeqpTpH23) pi denotes partial pressure of species " i ", and CMCH is the concentration of MCH. k=3.351024(slbcatft3)eE/RT E=70.0kcal/mol The ideal gas law is valid. 1. At what temperature and fractional conversion of MCH does the reactor operate? 2. Based only on the slopes of the G(T) and R(T) curves, is the operating point stable or unstable
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


