Question: Problem II: An irreversible aqueous reaction gave 9 0 % conversion in a batch reactor at 4 0 C in 1 0 0 min and
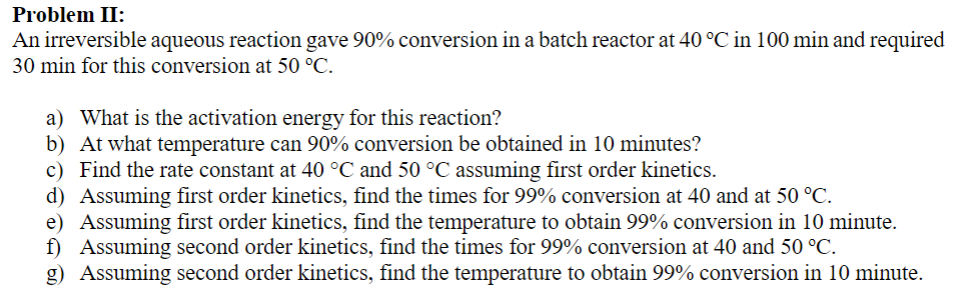
Problem II:
An irreversible aqueous reaction gave conversion in a batch reactor at in min and required
min for this conversion at
a What is the activation energy for this reaction?
b At what temperature can conversion be obtained in minutes?
c Find the rate constant at and assuming first order kinetics.
d Assuming first order kinetics, find the times for conversion at and at
e Assuming first order kinetics, find the temperature to obtain conversion in minute.
f Assuming second order kinetics, find the times for conversion at and
g Assuming second order kinetics, find the temperature to obtain conversion in minute.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


