Question: Problem Scenario A - Model Prediction and Analysis 2 1 An isothermal constant-volume batch reactor was used to carry out multiple runs of the liquid-phase
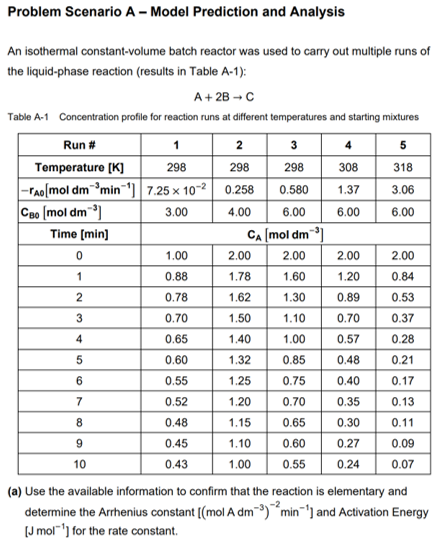
Problem Scenario A - Model Prediction and Analysis 2 1 An isothermal constant-volume batch reactor was used to carry out multiple runs of the liquid-phase reaction (results in Table A-1): A+2B-C Table A-1 Concentration profile for reaction runs at different temperatures and starting mixtures Run # 1 3 4 5 Temperature [K] 298 298 298 308 318 - Ao[mol dm min 1 7.25 x 107 0.258 0.580 1.37 3.06 Ceo mol dm) 3.00 4.00 6.00 6.00 6.00 Time [min] CA (mol dm) 0 1.00 2.00 2.00 2.00 2.00 0.88 1.78 1.60 1.20 0.84 2 0.78 1.62 1.30 0.89 0.53 3 0.70 1.50 1.10 0.70 0.37 4 0.65 1.40 1.00 0.57 0.28 5 0.60 1.32 0.85 0.48 0.21 0.55 1.25 0.75 0.40 0.17 0.52 1.20 0.70 0.35 0.13 8 0.48 1.15 0.65 0.30 0.45 1.10 0.60 0.27 0.09 10 0.43 1.00 0.55 0.24 0.07 (a) Use the available information to confirm that the reaction is elementary and determine the Arrhenius constant ((mol A dm-3) min') and Activation Energy (J mol"') for the rate constant. 6 7 0.11 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


