Question: 1. a. Which of the following components has the highest vapor pressure? b. Which of the components below dissolves best in hexane? Justify your
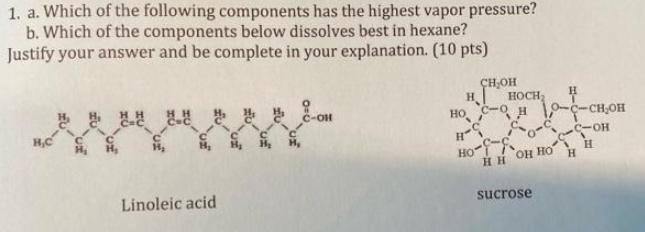
1. a. Which of the following components has the highest vapor pressure? b. Which of the components below dissolves best in hexane? Justify your answer and be complete in your explanation. (10 pts) H,C Linoleic acid C-OH CHOH H HOCH H - 10-C-CHOH H-S -- Ho H HH OH HO sucrose H
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Answer Linoleic acid has the highest vapor pressure of the two components This is because it is the lightest and has the lowest boiling point Sucrose ... View full answer

Get step-by-step solutions from verified subject matter experts


