Question: Problem One The experimental system shown in the figure ( bottom of page ) has two aqueous solutions in contact through a membrane, which is
Problem One
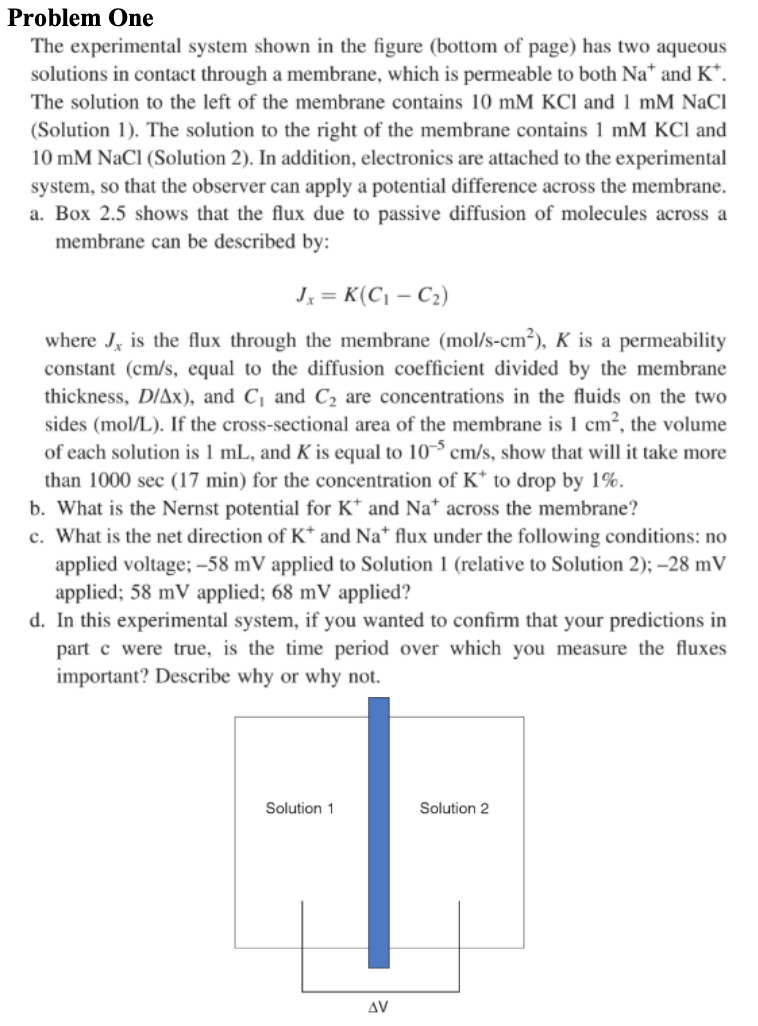
The experimental system shown in the figure bottom of page has two aqueous
solutions in contact through a membrane, which is permeable to both and
The solution to the left of the membrane contains and mMNaCl
Solution The solution to the right of the membrane contains and
mMNaCl Solution In addition, electronics are attached to the experimental
system, so that the observer can apply a potential difference across the membrane.
a Box shows that the flux due to passive diffusion of molecules across a
membrane can be described by:
where is the flux through the membrane is a permeability
constant equal to the diffusion coefficient divided by the membrane
thickness, and and are concentrations in the fluids on the two
sides If the crosssectional area of the membrane is the volume
of each solution is and is equal to show that will it take more
than for the concentration of to drop by
b What is the Nernst potential for and across the membrane?
c What is the net direction of and flux under the following conditions: no
applied voltage; applied to Solution relative to Solution ;
applied; applied; applied?
d In this experimental system, if you wanted to confirm that your predictions in
part c were true, is the time period over which you measure the fluxes
important? Describe why or why not.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


