Question: Problem Statement: Its is supposed that the carbon deposit C ( s ) is produced according to these following reactions: 2 CO ( g )
Problem Statement:
Its is supposed that the carbon deposit Cs is produced according to these following reactions:
COg Cs COgC
CHg CsHgD
Question Determine the minimum SC ratio molmol that can lead to carbon deposition from reaction C andor D
Question Determine which reaction from C or D that produces more carbon deposit, if any and if it happens
Assumptions:
a Reaction A and B always reach equilibrium
b The feed contains only methane and water vapor in gas phase
c Operating condition: bar and C
Notes:
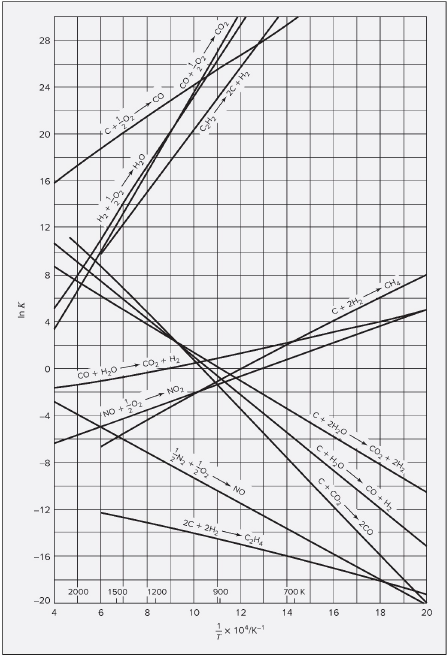
Attached is the equilibrium curve, I am able to retrieve all but one of the reactions please help finding the last k value for reaction A
For the second reaction, lnk k
For the third reaction, lnk k
For the fourth reaction, lnk k
NOTES: If you cannot answer the question, at least please attach the equilibrium chart that shows the k value for the first reaction steam reforming Just that and I will give thumbs up
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


