Question: 1 For the reaction 4A(g) + 3B(g) 2C(g) the following data were obtained at constant temp. Experiment Initial Rate Initial [A] Initial [B] No
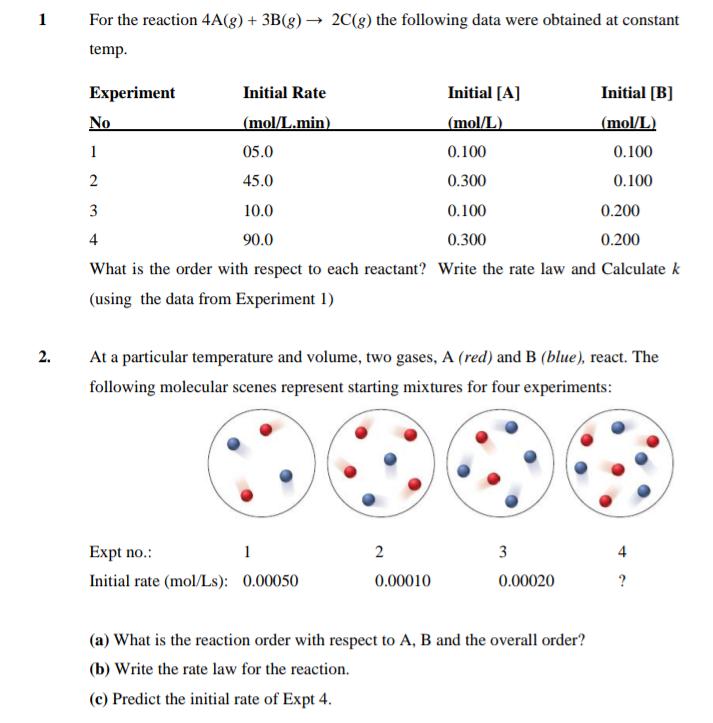
1 For the reaction 4A(g) + 3B(g) 2C(g) the following data were obtained at constant temp. Experiment Initial Rate Initial [A] Initial [B] No (mol/L.min) (mol/L) (mol/L) 1 05.0 0.100 0.100 2 45.0 0.300 0.100 3 10.0 0.100 0.200 4 90.0 0.300 0.200 What is the order with respect to each reactant? Write the rate law and Calculate k (using the data from Experiment 1) 2. At a particular temperature and volume, two gases, A (red) and B (blue), react. The following molecular scenes represent starting mixtures for four experiments: Expt no.: 1 2 3 4 Initial rate (mol/Ls): 0.00050 0.00010 0.00020 ? (a) What is the reaction order with respect to A, B and the overall order? (b) Write the rate law for the reaction. (c) Predict the initial rate of Expt 4.
Step by Step Solution
There are 3 Steps involved in it
1 2 Hello Student I ... View full answer

Get step-by-step solutions from verified subject matter experts


