Question: Problema 1 The elementary irreversible gas-phase reaction (A B + C) is carried out adiabatically in a PFR packed with a catalyst. Pure A enters
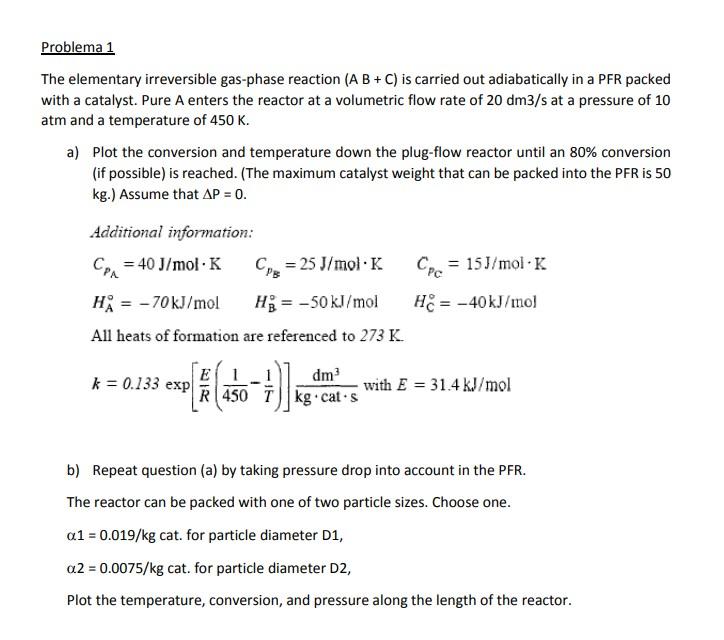
Problema 1 The elementary irreversible gas-phase reaction (A B + C) is carried out adiabatically in a PFR packed with a catalyst. Pure A enters the reactor at a volumetric flow rate of 20 dm3/s at a pressure of 10 atm and a temperature of 450 K. a) Plot the conversion and temperature down the plug-flow reactor until an 80% conversion (if possible) is reached. (The maximum catalyst weight that can be packed into the PFR is 50 kg.) Assume that AP = 0. Additional information: Ca = 40 J/mol K Cyg = 25 J/mol K = 15J/mol K Hi = -70kJ/mol Hg = -50 kJ/mol He = -40kJ/mol All heats of formation are referenced to 273 K. Cpc = k = 0.133 exp E dm with E = 31.4 kJ/mol R450 Tkg cats b) Repeat question (a) by taking pressure drop into account in the PFR. The reactor can be packed with one of two particle sizes. Choose one. a1 = 0.019/kg cat. for particle diameter D1, 0.2 = 0.0075/kg cat. for particle diameter D2, Plot the temperature, conversion, and pressure along the length of the reactor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


