Question: The elementary, irreversible gas-phase reaction A B + C Is carried out adiabatically in a PFR packed with a catalyst. Pure A enters the reactor
The elementary, irreversible gas-phase reaction
A B + C
Is carried out adiabatically in a PFR packed with a catalyst. Pure A enters the reactor at a volumetric flow rate of 20 dm3/sec, at a pressure of 10 atm, and a temperature of 450 K.
Additional information:
CPA = 40 J/molK CPB = 25 J/molK CPC = 15 J/molK
HA = -70 kJ/mol HB = -50 kJ/mol HC = -40 kJ/mol
All heats of formation are referenced to 273 K.
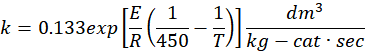
With E = 31.4 kJ/mol
- Plot and then analyze the conversion and temperature down the plug-flow reactor until and 80% conversion (if possible) is reached. (The maximum catalyst weight that can be packed into the PFR is 50 kg.) In other words plot X and T vs. catalyst weight. Assume the DP = 0.0.
k=0.133exp[RE(4501T1)]kgcatsecdm3 k=0.133exp[RE(4501T1)]kgcatsecdm3
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


