Question: Process 1 Process 2 P= const V = const T2 In the two processes shown in the figure aside, the same amount of heat, Q,
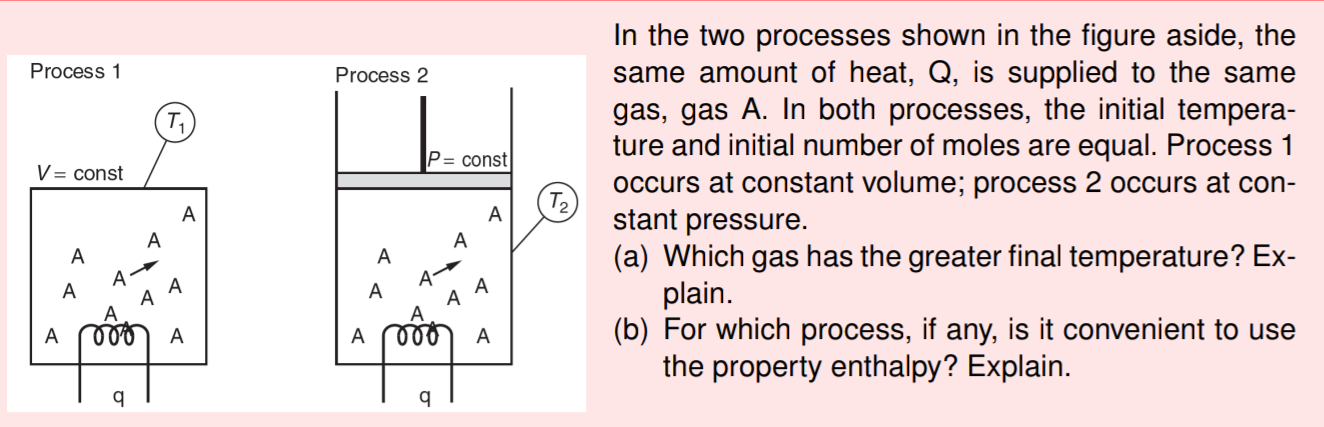
Process 1 Process 2 P= const V = const T2 In the two processes shown in the figure aside, the same amount of heat, Q, is supplied to the same gas, gas A. In both processes, the initial tempera- ture and initial number of moles are equal. Process 1 occurs at constant volume; process 2 occurs at con- stant pressure. (a) Which gas has the greater final temperature? Ex- plain. (b) For which process, if any, is it convenient to use the property enthalpy? Explain. A A A A A A A A A A A A A 000 A A 000 A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


