Question: Process Systems Analysis and Control problem 5.5 Consider the Continuous Flow Stirring Reactor (CSTR) given in Figure P5-5. The following reaction occurs in the reactor,
Process Systems Analysis and Control problem 5.5
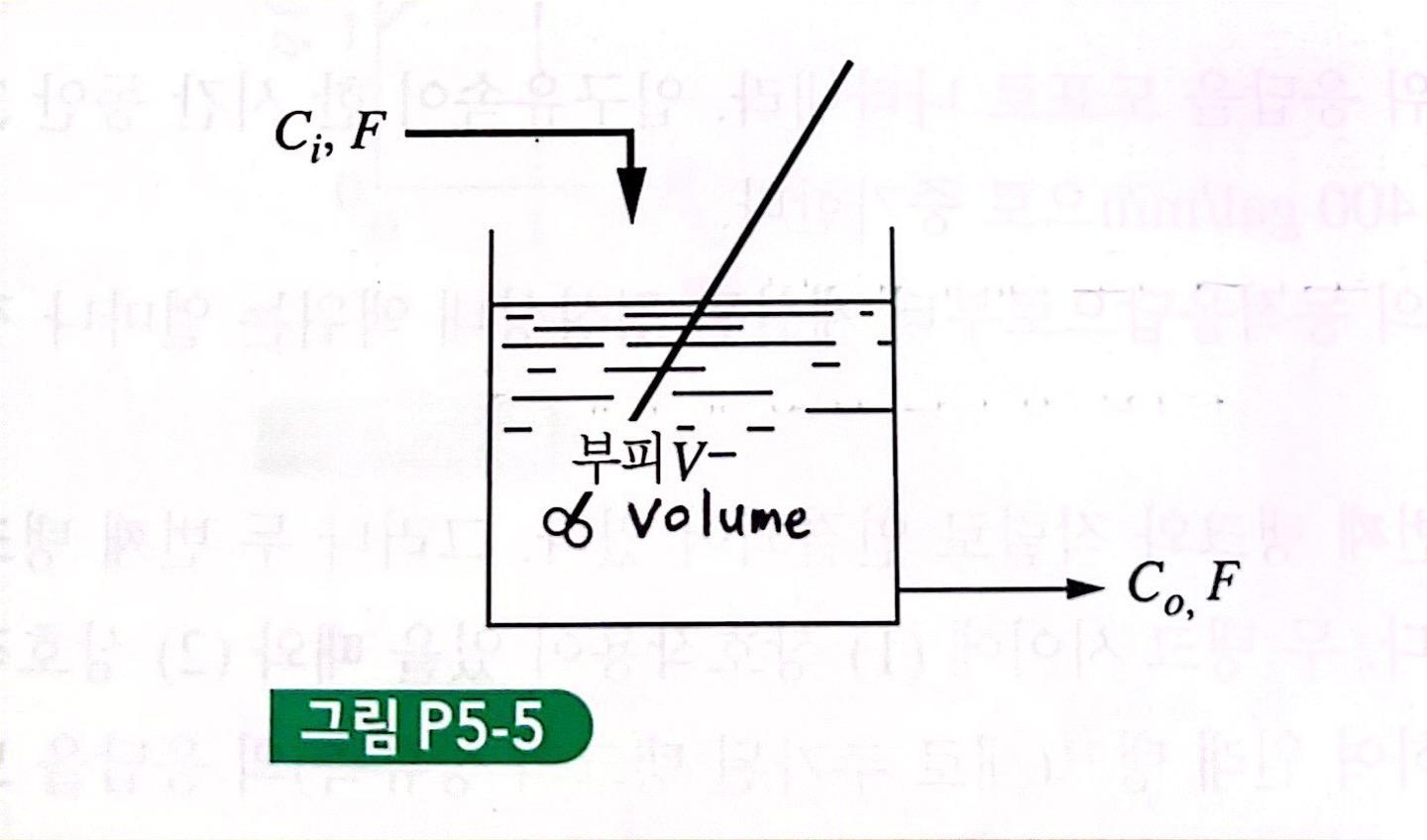
Consider the Continuous Flow Stirring Reactor (CSTR) given in Figure P5-5. The following reaction occurs in the reactor, AB The reaction rate equation is as follows. r = kC0 r = (moles of responding A)/(volume)(hours) k = Response rate constant C0(t) = Concentration of A in the reactor at time t (mole of A/volume) V = Volume of mixture in reactor F = feed rate of reactants , volume/time Ci(t) = Concentration of A in the feed, mole/volume (a) Assuming a constant density and a constant volume V, derive a transfer function that relates the concentration in the reactor to the concentration of the feed. (b) Write a block diagram for the reactor. (c) Graph the response of the reactor to the unit step input introduced into Ci.
P5-5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


