Question: Propionic acid in an aqueous solution is produced according to the reaction: C2H5COONa + HCL CH3COOH + NaCl In a laboratory test, equimolar quantities of
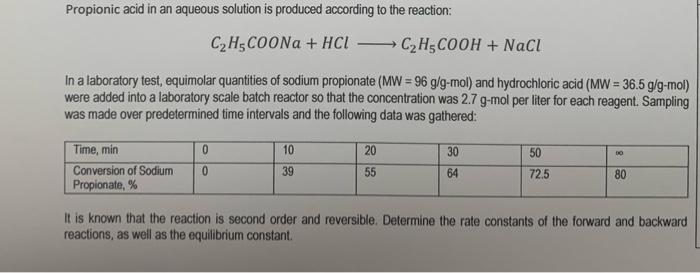
Propionic acid in an aqueous solution is produced according to the reaction: C2H5COONa + HCL CH3COOH + NaCl In a laboratory test, equimolar quantities of sodium propionate (MW = 96 g/g-mol) and hydrochloric acid (MW = 36.5 g/9-mol) were added into a laboratory scale batch reactor so that the concentration was 2.7 g-mol per liter for each reagent. Sampling was made over predetermined time intervals and the following data was gathered: 30 Time, min Conversion of Sodium Propionate, % 0 0 10 39 20 55 50 72.5 64 80 It is known that the reaction is second order and reversible. Determine the rate constants of the forward and backward reactions, as well as the equilibrium constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


