Question: Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave. Please I need the
Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave.
Please I need the algorithm, as it is written in the boldface. Thanks!!
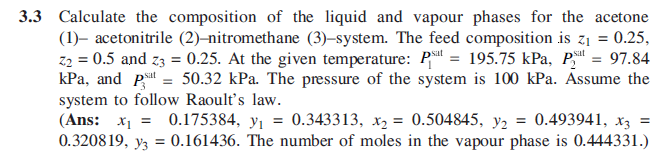
3.3 Calculate the composition of the liquid and vapour phases for the acetone (1), acetonitrile (2)-nitromethane (3)-system. The feed composition is z = 0.25, Z2 = 0.5 and zz = 0.25. At the given temperature: p = 195.75 kPa, P. = 97.84 kPa, and p = 50.32 kPa. The pressure of the system is 100 kPa. Assume the system to follow Raoult's law. (Ans: x1 = 0.175384, y = 0.343313, x2 = 0.504845, y2 = 0.493941, x3 = 0.320819, yz = 0.161436. The number of moles in the vapour phase is 0.444331.) =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


