Question: Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave. Please I need the
Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave.
Please I need the algorithm, as it is written in the boldface. Thanks!!
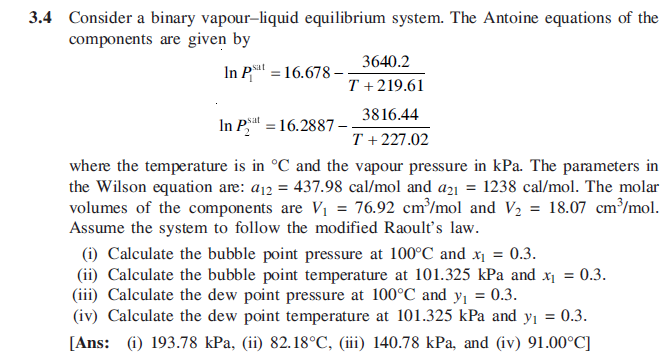
3.4 Consider a binary vapour-liquid equilibrium system. The Antoine equations of the components are given by 3640.2 In PS! = 16.678 - T + 219.61 3816.44 In P. = 16.2887 T + 227.02 where the temperature is in C and the vapour pressure in kPa. The parameters in the Wilson equation are: 812 = 437.98 cal/mol and a21 = 1238 cal/mol. The molar volumes of the components are V1 = 76.92 cm /mol and V2 = 18.07 cm /mol. Assume the system to follow the modified Raoult's law. (i) Calculate the bubble point pressure at 100C and x1 = 0.3. (ii) Calculate the bubble point temperature at 101.325 kPa and x = 0.3. (iii) Calculate the dew point pressure at 100C and y = 0.3. (iv) Calculate the dew point temperature at 101.325 kPa and y = 0.3. [Ans: (i) 193.78 kPa, (ii) 82.18C, (iii) 140.78 kPa, and (iv) 91.00C] = 3.4 Consider a binary vapour-liquid equilibrium system. The Antoine equations of the components are given by 3640.2 In PS! = 16.678 - T + 219.61 3816.44 In P. = 16.2887 T + 227.02 where the temperature is in C and the vapour pressure in kPa. The parameters in the Wilson equation are: 812 = 437.98 cal/mol and a21 = 1238 cal/mol. The molar volumes of the components are V1 = 76.92 cm /mol and V2 = 18.07 cm /mol. Assume the system to follow the modified Raoult's law. (i) Calculate the bubble point pressure at 100C and x1 = 0.3. (ii) Calculate the bubble point temperature at 101.325 kPa and x = 0.3. (iii) Calculate the dew point pressure at 100C and y = 0.3. (iv) Calculate the dew point temperature at 101.325 kPa and y = 0.3. [Ans: (i) 193.78 kPa, (ii) 82.18C, (iii) 140.78 kPa, and (iv) 91.00C] =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


