Question: Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave. Please I need the
Propose a solution algorithm for the problems below (Solution Flowchart), including all necessary equations, and implement the proposed algorithm in octave.
Please I need the algorithm, as it is written in the boldface. Thanks!!
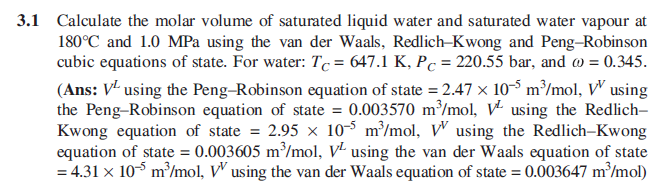
3.1 Calculate the molar volume of saturated liquid water and saturated water vapour at 180C and 1.0 MPa using the van der Waals, Redlich-Kwong and Peng-Robinson cubic equations of state. For water: Tc = 647.1 K, Pc = 220.55 bar, and 0 = 0.345. (Ans: V- using the Peng-Robinson equation of state = 2.47 x 10-5 m/mol, V using the Peng-Robinson equation of state = 0.003570 m /mol, VI using the Redlich- Kwong equation of state = 2.95 x 10-5 m?/mol, V using the Redlich-Kwong equation of state = 0.003605 m/mol, VI using the van der Waals equation of state = 4.31 x 10- m/mol, V using the van der Waals equation of state = 0.003647 m/mol)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


