Question: Provide complete solutions and explain thoroughly. 4. The gure shows a cycle undergone by 1.00 mol of an ideal ' monatomic gas. The temperatures are
Provide complete solutions and explain thoroughly.
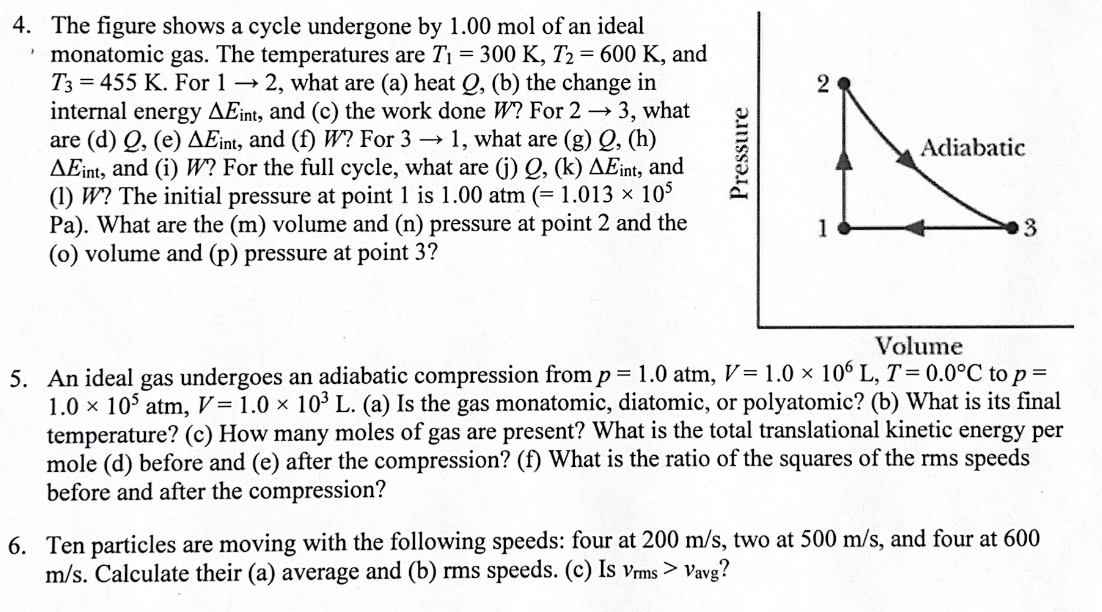
4. The gure shows a cycle undergone by 1.00 mol of an ideal ' monatomic gas. The temperatures are T1 = 300 K, T2 = 600 K, and T3 = 455 K. For 1 + 2, what are (a) heat Q, (b) the change in internal energy Mint, and (c) the work done W? For 2 a 3, what are (d) Q, (e) AEim, and (t) W? For 3 > 1, what are (g) Q, (h) Mint, and (i) W? For the full cycle, what are (i) Q, (1:) Mint, and (l) W? The initial pressure at point 1 is 1.00 atm (= 1.013 > vavg
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


