Question: Provide complete solutions and explain thoroughly. Also, don't forget to explain the reason for the #5 a) and #6 c) answers. 5. An ideal gas
Provide complete solutions and explain thoroughly. Also, don't forget to explain the reason for the #5 a) and #6 c) answers.
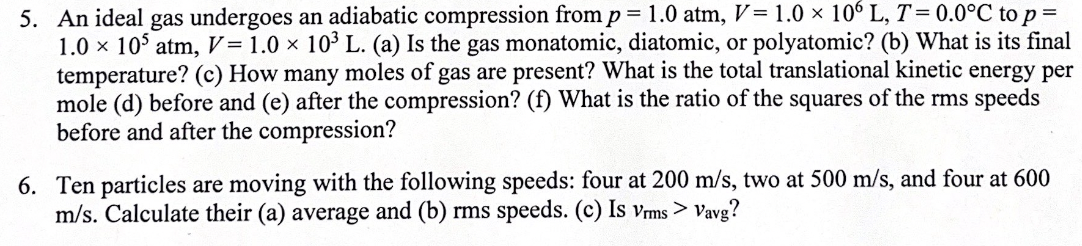
5. An ideal gas undergoes an adiabatic compression from p = 1.0 atm, V = 1.0 x 10% L, T= 0.0 C to p = 1.0 x 10' atm, V = 1.0 x 10' L. (a) Is the gas monatomic, diatomic, or polyatomic? (b) What is its final temperature? (c) How many moles of gas are present? What is the total translational kinetic energy per mole (d) before and (e) after the compression? (f) What is the ratio of the squares of the rms speeds before and after the compression? 6. Ten particles are moving with the following speeds: four at 200 m/s, two at 500 m/s, and four at 600 m/s. Calculate their (a) average and (b) rms speeds. (c) Is Vis > Vavg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


