Question: provided box. Remember to enter your answer to the correct number of significant figures. For the reaction: Ag) +B(g) AB(g) the rate is 0.31 mol/L's,
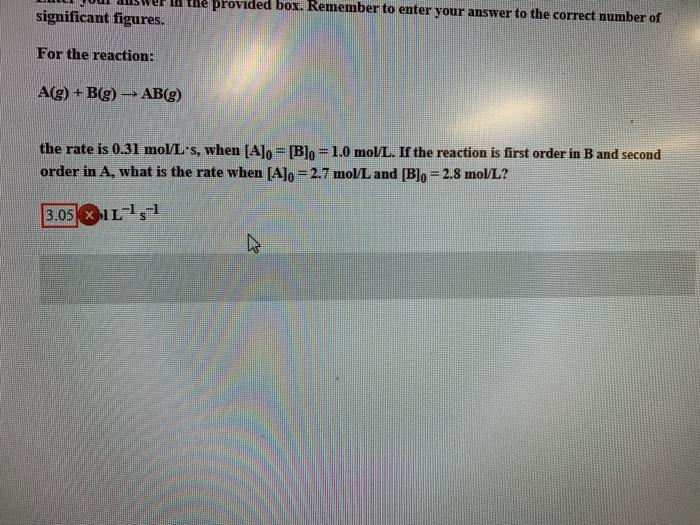
provided box. Remember to enter your answer to the correct number of significant figures. For the reaction: Ag) +B(g) AB(g) the rate is 0.31 mol/L's, when [A]o= [Blo=1.0 mol/L. If the reaction is first order in B and second order in A, what is the rate when [Alo = 2.7 mol/L and [Blo = 2.8 mol/L? 3.05 XL 1-1 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


