Question: 18 3 attempts loft Check my work Enter your answer in the provided box. Remember to enter your answer to the correct number of significant
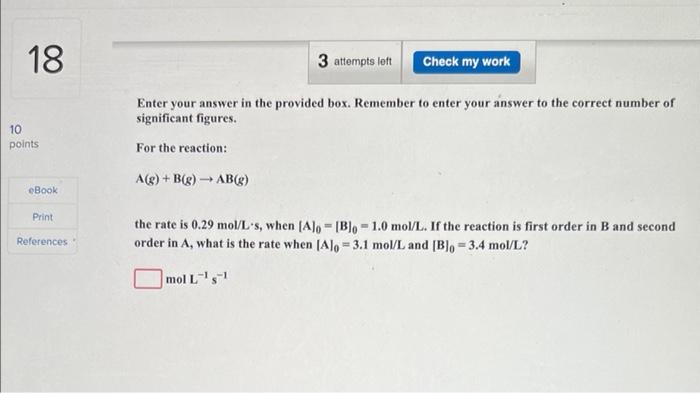
18 3 attempts loft Check my work Enter your answer in the provided box. Remember to enter your answer to the correct number of significant figures. 10 points For the reaction: A(g) + B(8) AB(8) eBook Print References the rate is 0.29 mol/L's, when (Alo - 1B). - 1.0 mol/L. If the reaction is first order in B and second order in A, what is the rate when (Alo = 3.1 mol/L and [B)- 3.4 mol/L? mol L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


