Question: Saved Check my work Enter your answer in the provided box. Remember to enter your answer to the correct number of significant figures. For the
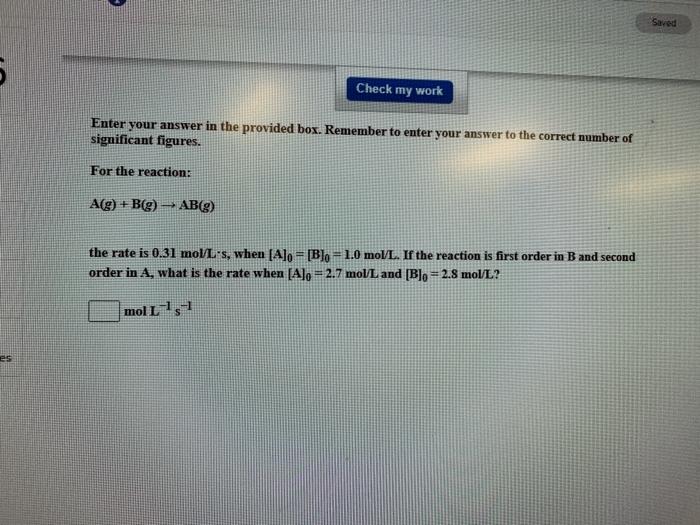
Saved Check my work Enter your answer in the provided box. Remember to enter your answer to the correct number of significant figures. For the reaction: A(g) + B(g) AB(g) the rate is 0.31 mol L-s, when [Alo - [B]o - 1.0 moVL. If the reaction is first order in B and second order in A, what is the rate when [Alo = 2.7 mol/L and [Blo= 2.8 mol/L? mol L 1,7 es
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


