Question: Put the following steps in the correct order for determining the presence of NH4+ion in a solid unknown The smell of the gas evolved is
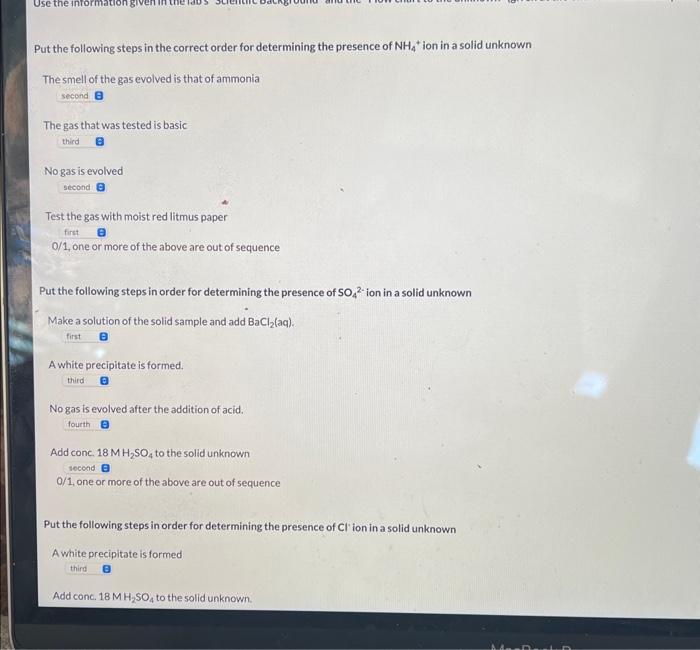
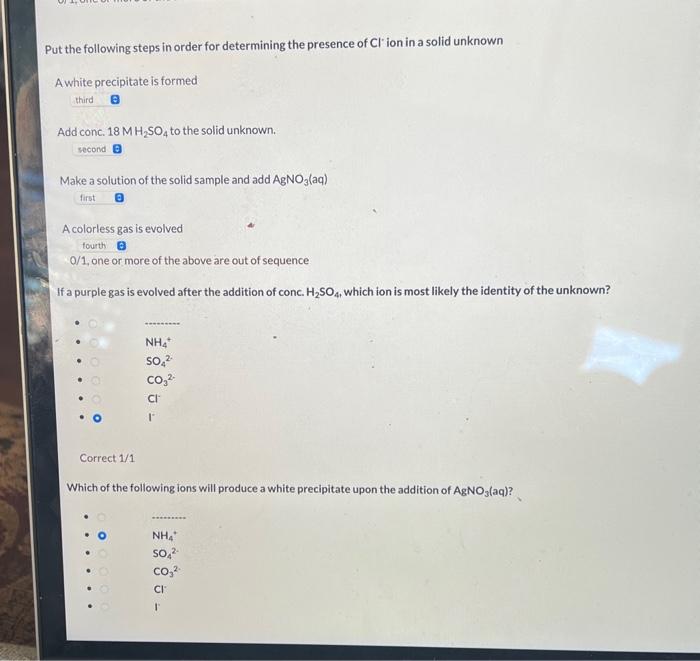

Put the following steps in the correct order for determining the presence of NH4+ion in a solid unknown The smell of the gas evolved is that of ammonia The gas that was tested is basic No gas is evolved Test the gas with moist red litmus paper 0/1, one or more of the above are out of sequence Put the following steps in order for determining the presence of SO42 ion in a solid unknown Make a solution of the solid sample and add BaCl2(aq). A white precipitate is formed. No gas is evolved after the addition of acid. Add conc. 18MH2SO4 to the solid unknown 0/1, one or more of the above are out of sequence Put the following steps in order for determining the presence of Cl ' ion in a solid unknown A white precipitate is formed Make a solution of the solid sample and add AgNO3(aq) A colorless gas is evolved 0/1, one or more of the above are out of sequence If a purple gas is evolved after the addition of conc. H2SO4, which ion is most likely the identity of the unknown? Correct 1/1 Which of the following ions will produce a white precipitate upon the addition of AgNO3(aq) ? Which of the following ions will produce a white precipitate upon the addition of AgNO3(aq) ? NH4+ - SO42 - CO32 Cl - r Incorrect 0/1 Which of the following ions will produce a yellow precipitate upon the addition of AgNO3(aq) ? - NH4+ - SO42 - CO32. - Cl - 1 Incorrect 0/1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


