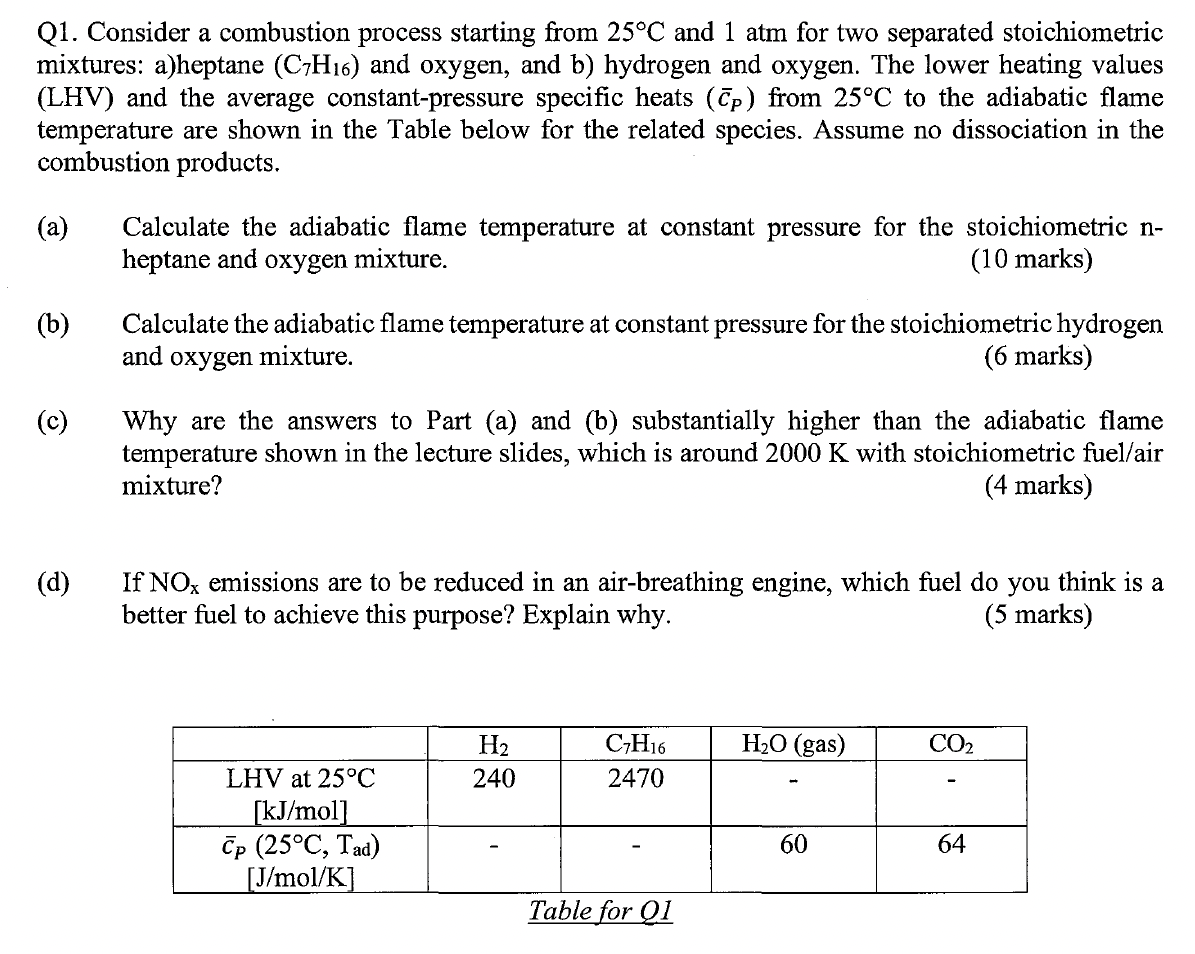
Question: Q 1 . Consider a combustion process starting from ( 2 5 ^ { circ } mathrm { C } )
Q Consider a combustion process starting from circmathrmC and atm for two separated stoichiometric mixtures: aheptane leftmathrmCmathrmHright and oxygen, and b hydrogen and oxygen. The lower heating values LHV and the average constantpressure specific heats leftbarcPright from circmathrmC to the adiabatic flame temperature are shown in the Table below for the related species. Assume no dissociation in the combustion products.
a Calculate the adiabatic flame temperature at constant pressure for the stoichiometric nheptane and oxygen mixture.
marks
b Calculate the adiabatic flame temperature at constant pressure for the stoichiometric hydrogen and oxygen mixture.
marks
c Why are the answers to Part a and b substantially higher than the adiabatic flame temperature shown in the lecture slides, which is around K with stoichiometric fuelair mixture?
marks
d If mathrmNOx emissions are to be reduced in an airbreathing engine, which fuel do you think is a better fuel to achieve this purpose? Explain why.
marks
Table for QI
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


