Question: Q: Balance each redox equation by half reaction method. 12 + ClO3+ (aq) 103 (aq) + Cl(aq) (acidic conditions) 2- 52- (aq) + Br2 +504-
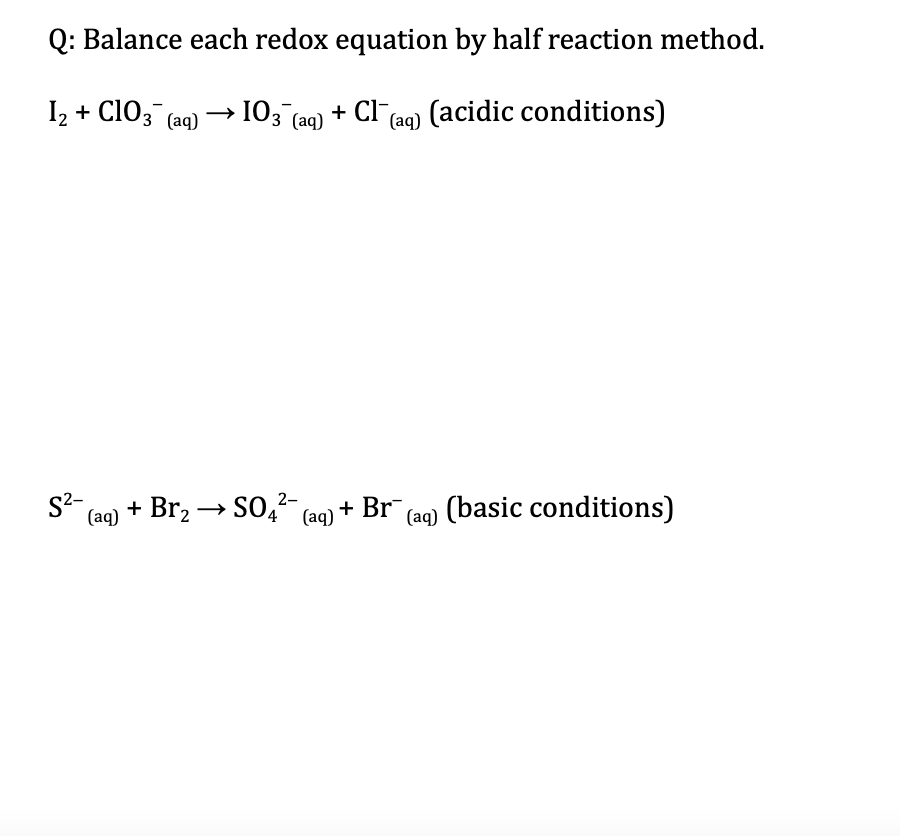
Q: Balance each redox equation by half reaction method. 12 + ClO3+ (aq) 103 (aq) + Cl"(aq) (acidic conditions) 2- 52- (aq) + Br2 +504- (aq) + Br (aq) (basic conditions) (
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


