Question: Q1. Consider a CSTR with two reactions (isothermal with first order kinetics) to handle multiple reactions (assume a constant volume reactor). A+B2P 2A+P+Q (reaction 1)
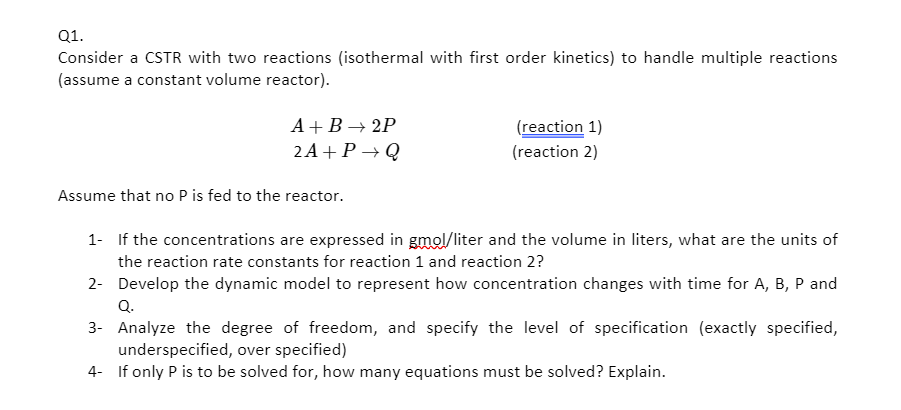
Q1. Consider a CSTR with two reactions (isothermal with first order kinetics) to handle multiple reactions (assume a constant volume reactor). A+B2P 2A+P+Q (reaction 1) (reaction 2) Assume that no Pis fed to the reactor. 1- If the concentrations are expressed in gmol/liter and the volume in liters, what are the units of the reaction rate constants for reaction 1 and reaction 2? 2- Develop the dynamic model to represent how concentration changes with time for A, B, P and Q. 3- Analyze the degree of freedom, and specify the level of specification (exactly specified, underspecified, over specified) 4- If only P is to be solved for, how many equations must be solved? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


