Question: Q1. Consider a CSTR with two reactions (isothermal with first order kinetics) to handle multiple reactions (assume a constant volume reactor). A+B_2P (reaction 1) 2A+
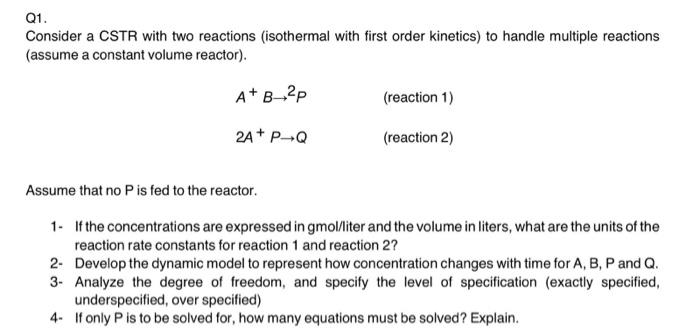
Q1. Consider a CSTR with two reactions (isothermal with first order kinetics) to handle multiple reactions (assume a constant volume reactor). A+B_2P (reaction 1) 2A+ P4Q (reaction 2) Assume that no Pis fed to the reactor. 1. If the concentrations are expressed in gmol/liter and the volume in liters, what are the units of the reaction rate constants for reaction 1 and reaction 2? 2- Develop the dynamic model to represent how concentration changes with time for A, B, P and Q. 3. Analyze the degree of freedom, and specify the level of specification (exactly specified, underspecified, over specified) 4. If only Pis to be solved for, how many equations must be solved? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


