Question: Q3: (20 points) The first three diffraction angles of the XRD pattem are observed using Cu K alphs (0.14506 nm) at 20 = 38.6,
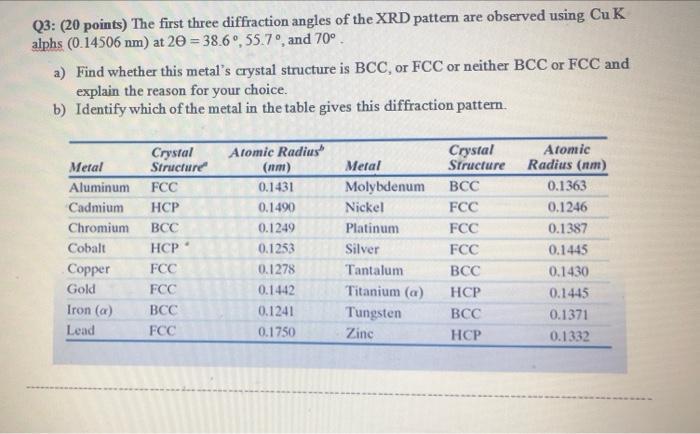
Q3: (20 points) The first three diffraction angles of the XRD pattem are observed using Cu K alphs (0.14506 nm) at 20 = 38.6, 55.7, and 70. a) Find whether this metal's crystal structure is BCC, or FCC or neither BCC or FCC and explain the reason for your choice. b) Identify which of the metal in the table gives this diffraction pattern. Crystal Structure Metal Aluminum FCC Cadmium HCP Chromium BCC HCP FCC FCC Cobalt Copper Gold Iron (a) Lead BCC FCC Atomic Radius (nm) 0.1431 0.1490 0.1249 0.1253 0.1278 0.1442 0.1241 0.1750 Metal Molybdenum Nickel Platinum Silver Tantalum Titanium (a) Tungsten Zinc Crystal Structure BCC FCC FCC FCC BCC HCP BCC HCP Atomic Radius (nm) 0.1363 0.1246 0.1387 0.1445 0.1430 0.1445 0.1371 0.1332
Step by Step Solution
3.33 Rating (147 Votes )
There are 3 Steps involved in it
20 36 557 700 sin 0 109 ... View full answer

Get step-by-step solutions from verified subject matter experts


