Question: Q3. Consider the following five point calibration plot using absorbance and concentration. 0.8 0.7 0.6 0.5 0.4 0.3 y = 0,4508x +0.0444 R=0.9876 0.2
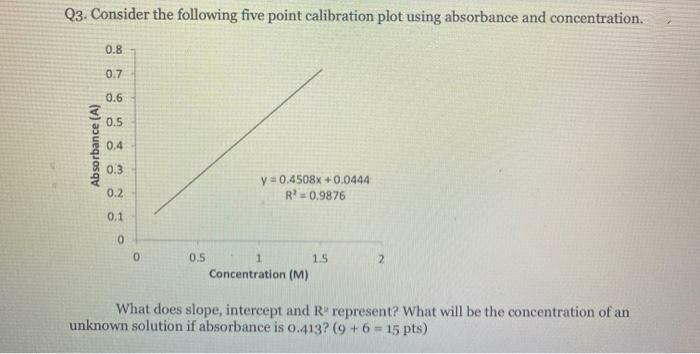
Q3. Consider the following five point calibration plot using absorbance and concentration. 0.8 0.7 0.6 0.5 0.4 0.3 y = 0,4508x +0.0444 R=0.9876 0.2 0.1 0.5 1. 1.5 2. Concentration (M) What does slope, intercept and R" represent? What will be the concentration of an unknown solution if absorbance is o.413? (9 +6 -15 pts) Absorbance (A)
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
Here slope represents the steepness of line when the line crosses y axis then this point i... View full answer

Get step-by-step solutions from verified subject matter experts


