Question: Question 1 (2 points) Fill in the blank. Use only the number in numerical form. Based on the Bohr model, this atom has valence electrons.
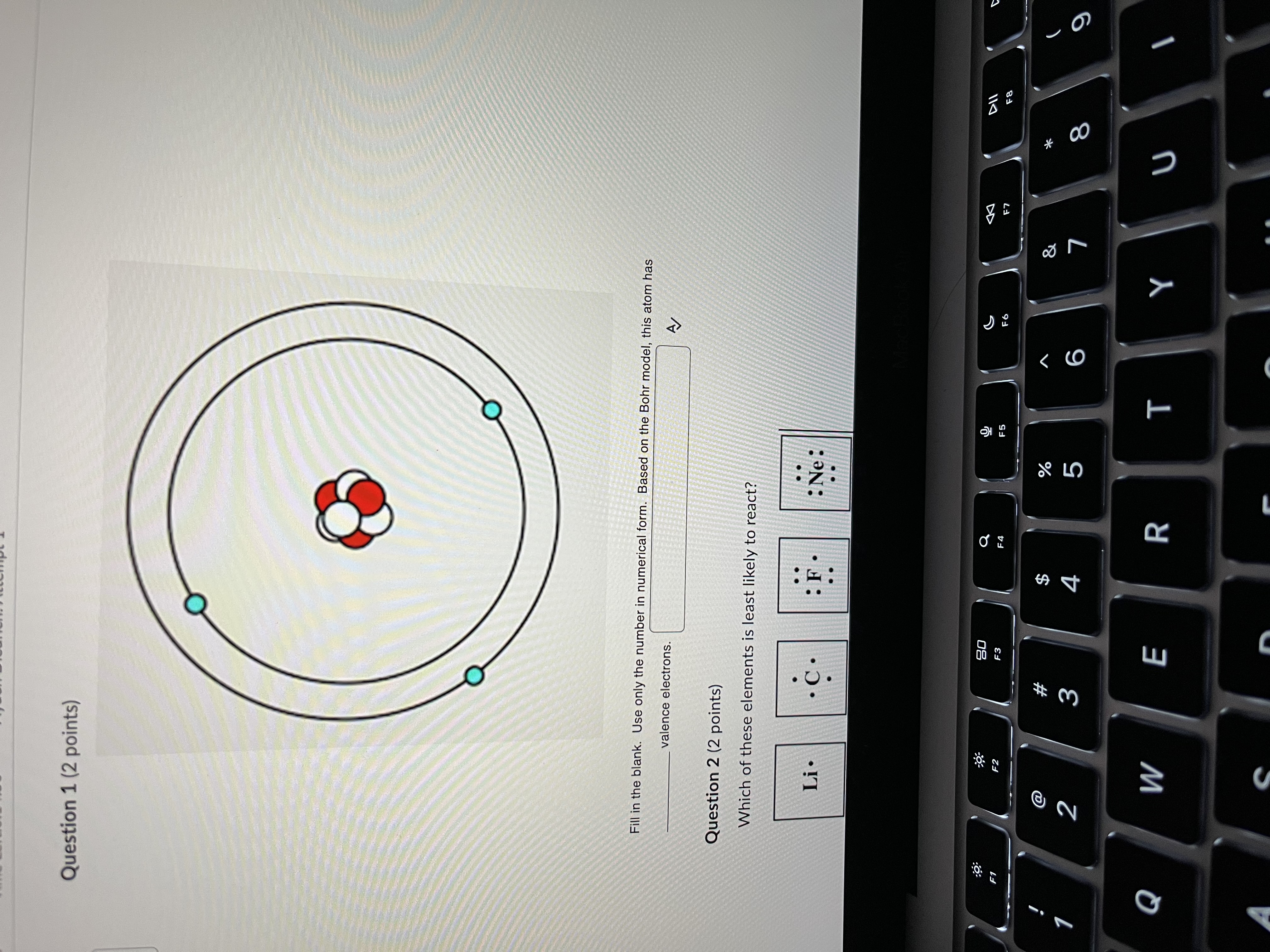
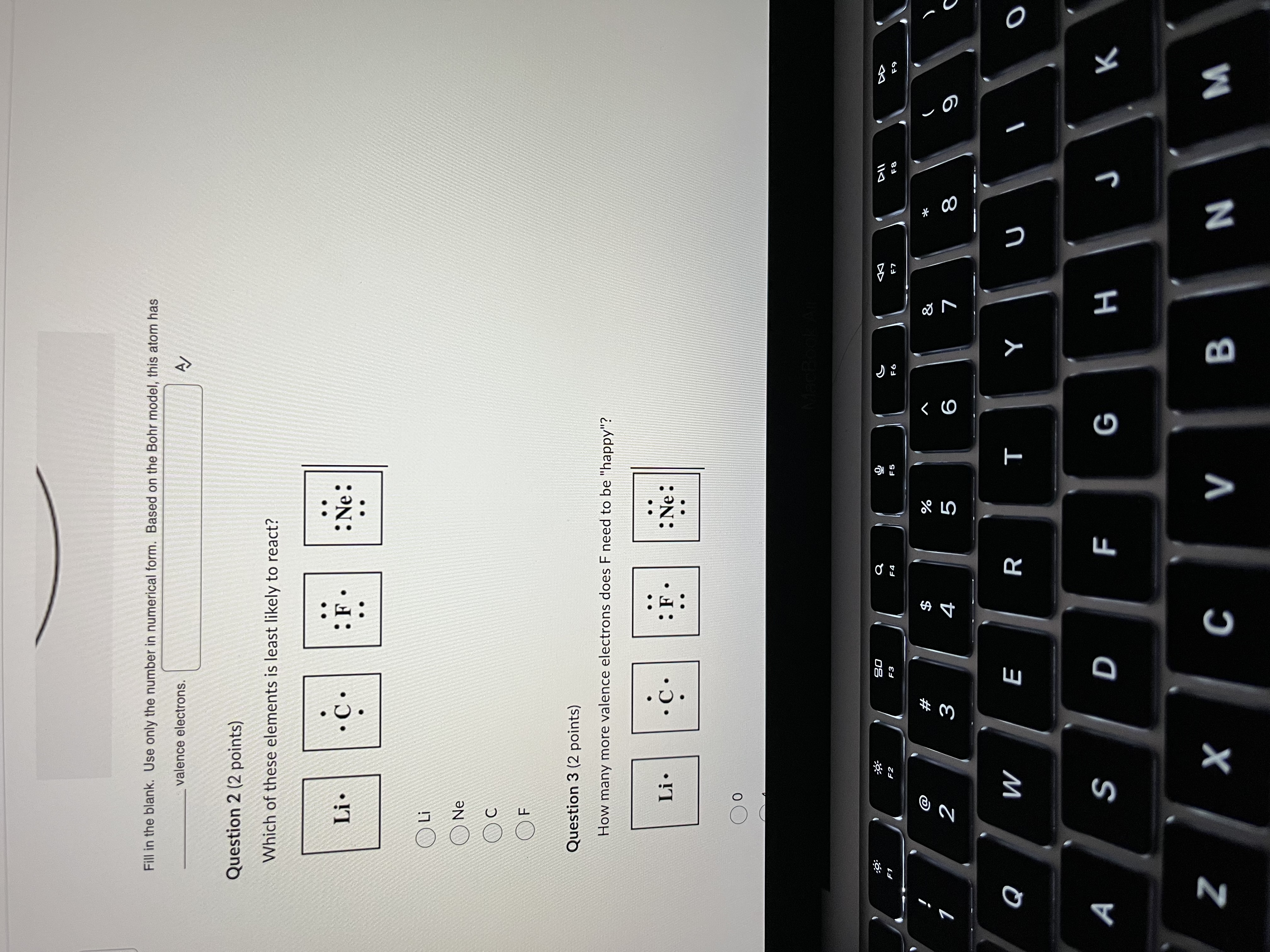
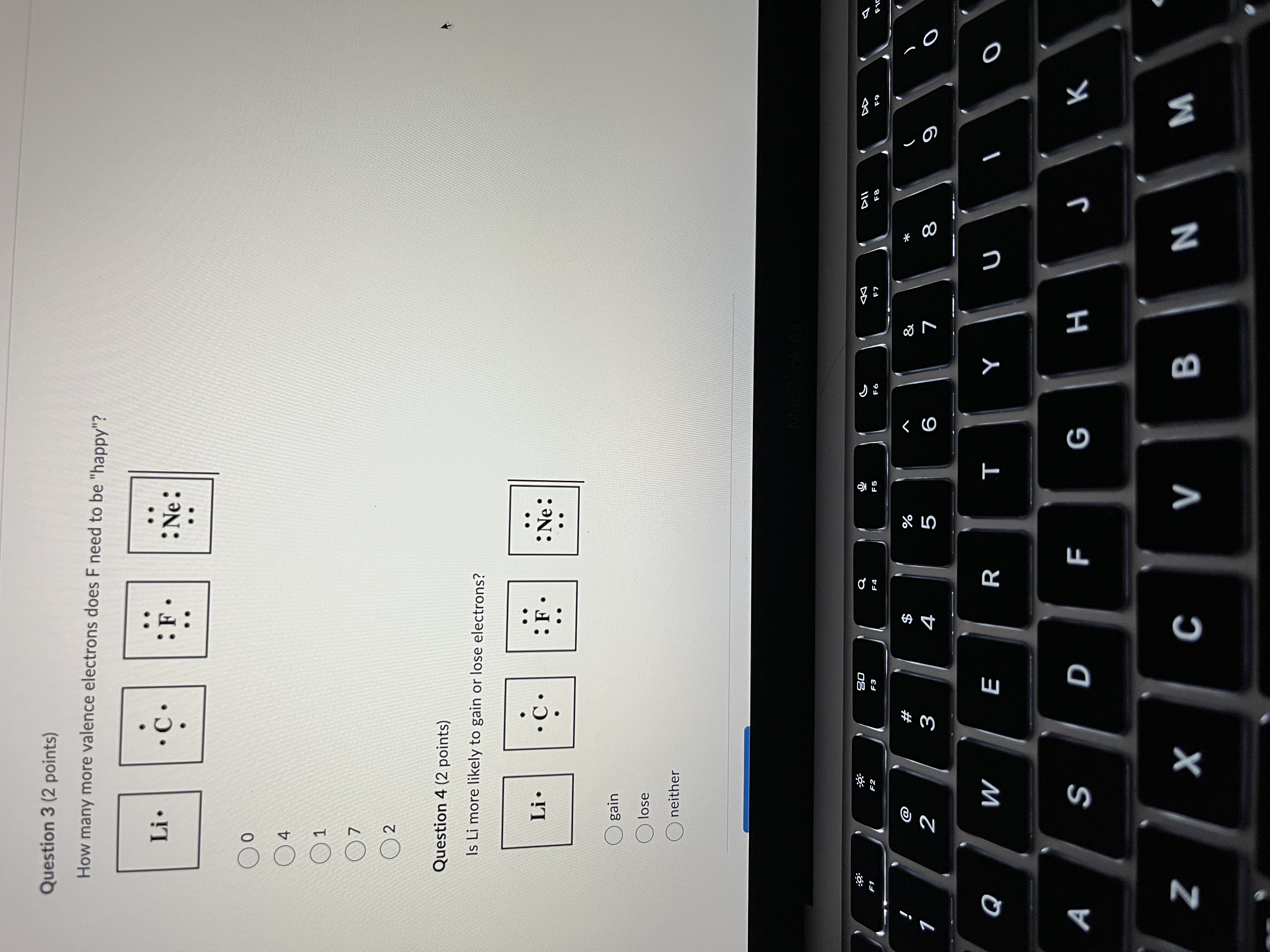
Question 1 (2 points) Fill in the blank. Use only the number in numerical form. Based on the Bohr model, this atom has valence electrons. Question 2 (2 points) Which of these elements is least likely to react? Li . : Ne: :9 30 a F1 E2 F3 F6 F7 F8 % & W# NO V 5 6 7 8 9 Q W E R T Y UFill in the blank. Use only the number in numerical form. Based on the Bohr model, this atom has valence electrons. Question 2 (2 points) Which of these elements is least likely to react? Li. C . : F : Ne: . . OLi Ne Oc OF Question 3 (2 points) How many more valence electrons does F need to be "happy"? Li . C . : Ne: Oo :9 20 @ #F D N 3 5 6 O Q W E R T Y U O A S D F G H J K Z X C V B N MQuestion 3 (2 points) How many more valence electrons does F need to be "happy"? Li . : Ne: Question 4 (2 points) Is Li more likely to gain or lose electrons? Li . : Ne: gain lose neither 63 S DII #F 2 W 5 6 9 O Q W E R T Y U O A S D F G H J K Z X C V B N M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


