Question: Question #1 Determine the requested quantities for the following questions. Report your answer to 3 decimal places (a) pH of 0.04472 M acetic acid (HAC)?
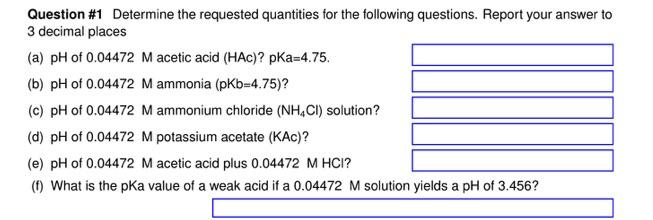
Question #1 Determine the requested quantities for the following questions. Report your answer to 3 decimal places (a) pH of 0.04472 M acetic acid (HAC)? Ka=4.75. (b) pH of 0.04472 M ammonia (pKb=4.75)? (c) pH of 0.04472 M ammonium chloride (NH,CI) solution? (d) pH of 0.04472 M potassium acetate (KAc)? (e) pH of 0.04472 M acetic acid plus 0.04472 M HCI? (0) What is the pka value of a weak acid if a 0.04472 M solution yields a pH of 3.456
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


