Question: Question 11 6 Points (A) If a 0.3 M solution of NaOH was used to titrate 100 mL of 0.3 M solution of CH2COOH (Ka


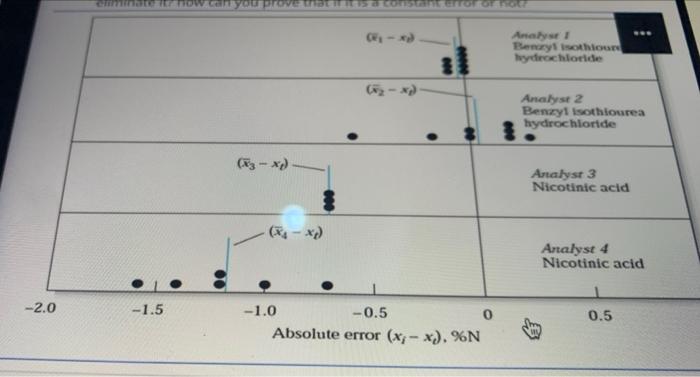
Question 11 6 Points (A) If a 0.3 M solution of NaOH was used to titrate 100 mL of 0.3 M solution of CH2COOH (Ka =1.8 x 10-5) and the following volumes of NaOH were recorded: 102, 97,99, 98, 101, 106 mL. 1. The pH value is... ............wl added 99.0 mL of 0.3 M NaOH 2. The pH of the solution at the equivalence point more than 7 Because........ 3. Calculate the 99% confidence limits of the mean? And use them to decide whether there is any evidence of systematic error? [hints: For N=6 and the 99 % confidence interval, the value of t is 4.03] (B) Regarding to the Figure below, which summarizes the results for the determination of Nitrogen in two pure compounds: berlisothiourea hydrochloride and nicotinic acid. Answer the following questions: 1. Which of the analysts was precise but inaccurate? Why? 2. Why analysts 3 shows systematic errors of about -0.7 % Nitrogen? And is possible to eliminate it? how can you prove that if it is a constant error or not? BO 1 - x) 00000 Analyst 1 Benzyl isothiourea hydrochloride (x - x) now.com YouPVERDELE STORE - Analyse Bency isothion hydrochloride Analyse 2 Benzyl Isothiourea hydrochloride Analyst 3 Nicotinic acid (4- *) Analyst 4 Nicotinic acid -2.0 -1.5 0.5 -1.0 -0.5 Absolute error (x; - *). %N
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


