Question: Question 12 (5 points) Supercritical 73.0 Solid Liquid Pressure (atm) Gas 5.11 3L1 -785564 Temperature (C) Phase Diagram for Unknown Substance At a constant pressure
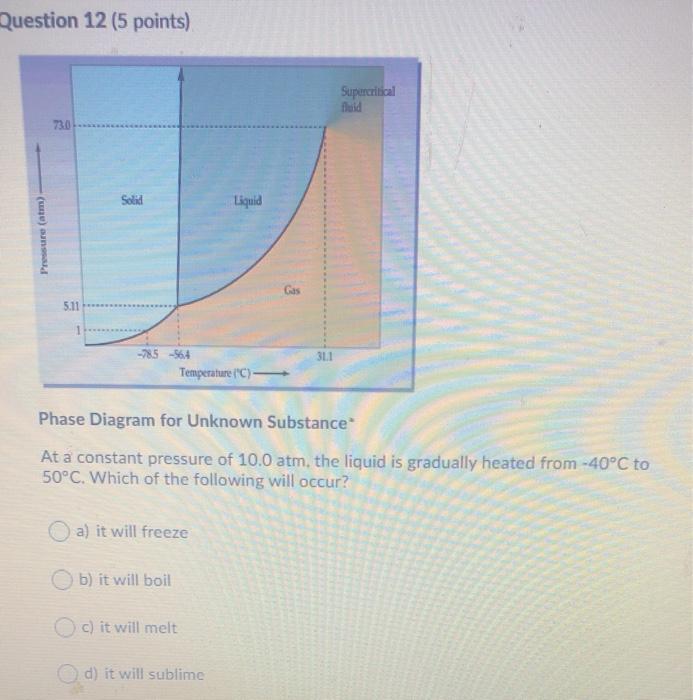
Question 12 (5 points) Supercritical 73.0 Solid Liquid Pressure (atm) Gas 5.11 3L1 -785564 Temperature (C) Phase Diagram for Unknown Substance At a constant pressure of 10.0 atm, the liquid is gradually heated from -40C to 50C. Which of the following will occur? a) it will freeze b) it will boil c) it will melt d) it will sublime
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


